��Ŀ����
15����ijһ�ݻ�Ϊ2L���ܱ������ڣ����� 0.12mol��CO��0.12mol��H2O���ڴ������ں�800��������¼��ȣ��������·�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0����Ӧ��CO2��Ũ����ʱ��仯������±���| ʱ�䣨min�� | 0 | 5 | 10 | 15 | 20 |
| c��CO2����mol/L�� | 0.00 | 0.02 | 0.03 | 0.03 | 0.03 |
��2�����������������£��ı�����������ʹƽ�ⳣ��������A
A�������¶� B�������¶� C��������� D���Ƴ�������̼����
��3����Ҫһ��ʼ����0.04mol��CO��0.04mol��H2O��0.08mol��CO2��0.08mol��H2������ͬ�������£���Ӧ��ƽ��ʱ��c��CO��=0.03mol/L��
��4���������¶Ⱥ�������������䣬�ڣ�1��������ƽ����ϵ�У��ٳ���0.12mol ��ˮ���������´ﵽƽ���CO��ת�������ߣ�����ߡ��������͡����ǡ����䡱����CO2�������������ͣ�����ߡ��������͡����ǡ����䡱����
��5���ڴ������ں�800��������£�����Ͷ�ϣ������ijһʱ��c��CO��=c��H2O��=0.09mol/L��c��CO2 ��=c��H2��=0.13mol/L�����ʱv��������v���棩���á���������������=������
���� ��1�����ݱ���CO2��Ũ�ȱ仯�ж�ƽ��״̬��Ȼ����ݶ�����̼��Ũ�ȱ仯���CO��Ũ�ȱ仯���ٸ���v=$\frac{��c}{��t}$���㷴Ӧ���ʣ�����ƽ�ⳣ��ָ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���н��
��2��ƽ�ⳣ��ֻ���¶ȵı仯���仯�������¶ȶ�ƽ���Ӱ�������
��3�����һ��ʼ����0.04mol��CO��0.04mol��H2O��0.08mol��CO2��0.08mol��H2���뿪ʼʱ����0.12mol��CO��0.12mol��H2O��Ϊ��Чƽ�⣬�����������£��ﵽƽ��״̬ʱ��CO�����ʵ���Ũ����ȣ�
��4�����淴Ӧ������һ�ַ�Ӧ���Ũ�ȣ�����һ�ַ�Ӧ���ת���ʻ���������ˮ���������ʵ���ʹ������������������ֻ����һ�ַ�Ӧ�����ƽ�������ƶ��ij̶Ⱥ�С����Ӧ�����ɵĶ�����̼���٣�������̼���������Ӳ������Ի�������ж�����̼�������������С��
��5������Qc=$\frac{c��C{O}_{2}��•c��{H}_{2}��}{c��CO��•c��{H}_{2}O��}$��ƽ�ⳣ��K�Ĵ�С��ϵ�жϣ�
��� �⣺��1����ijһ�ݻ�Ϊ5L���ܱ������ڣ�����0.3mol��CO��0.3mol��H2O������ʼŨ��c��CO��=0.06mol/L��c��H2O��=0.06mol/L��ƽ��ʱc��CO2��=0.03mol/L����
CO��g��+H2O��g��?CO2��g��+H2��g��
��ʼŨ��/mol•L-1 ��0.06 0.06 0 0
ת��Ũ��/mol•L-1 ��0.03 0.03 0.03 0.03
ƽ��Ũ��/mol•L-1 ��0.03 0.03 0.03 0.03
��Ӧ��ʼ���ﵽƽ��ʱ��ת����COΪc��CO��=0.03mol/L����v��CO��=$\frac{0.03mol/L}{10min}$=0.003mol/��L•min��-1��
���¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ��K=$\frac{c��C{O}_{2}��•c��{H}_{2}��}{c��CO��•c��{H}_{2}O��}$=$\frac{0.03��0.03}{0.03��0.03}$=1��
�ʴ�Ϊ��0.003��1��
��2��ƽ�ⳣ��ֻ���¶ȵı仯���仯����֪CO��g��+H2O��g��?CO2��g��+H2��g����H��0���������¶�ƽ�������ƶ���ƽ�ⳣ��K����
�ʴ�Ϊ��A��
��3�����һ��ʼ����0.04mol��CO��0.04mol��H2O��0.08mol��CO2��0.08mol��H2���뿪ʼʱ����0.12mol��CO��0.12mol��H2O��Ϊ��Чƽ�⣬�����������£��ﵽƽ��״̬ʱ��CO�����ʵ���Ũ����ȣ����Դﵽƽ��״̬ʱ��c��CO��=0.03mol/L��
�ʴ�Ϊ��0.03��
��4�����淴Ӧ������һ�ַ�Ӧ���Ũ�ȣ�����һ�ַ�Ӧ���ת���ʻ����������������¶Ⱥ�������������䣬�ڣ�1��������ƽ����ϵ�У��ٳ���0.12mol ��ˮ���������´ﵽƽ���CO��ת�������ߣ�
����ˮ���������ʵ���ʹ������������������ֻ����һ�ַ�Ӧ�����ƽ�������ƶ��ij̶Ⱥ�С����Ӧ�����ɵĶ�����̼���٣�������̼���������Ӳ������Ի�������ж�����̼�������������С��
�ʴ�Ϊ�����ߣ����ͣ�
��5���ڴ������ں�800��������£���ijһʱ�̲��C��CO��=C��H2O��=0.09mol/L��C��CO2 ��=C��H2��=0.13mol/L��
��ʱ��Ũ����Qc=$\frac{c��C{O}_{2}��•c��{H}_{2}��}{c��CO��•c��{H}_{2}O��}$=$\frac{0.13��0.13}{0.09��0.09}$=2.1��K=1����Ӧ��Ũ��ƫ�ߣ���ƽ�����������ƶ���v��������v���棩��
�ʴ�Ϊ������
���� ���⿼�黯ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ��漰��Ӧ���ʵļ��㡢��ѧƽ���йؼ��㡢��ѧƽ���ƶ���Ӱ�����ء�K��Ӧ�õ�֪ʶ��ע�����ƽ�ⳣ����Ӧ�á�ƽ���ƶ��ı��ʣ�����������ѧ���ķ�����������ѧ����������

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�| A�� | 150L2mol•L-1NaCl��Һ | B�� | 70.5L0.5mol•L-1CaCl2��Һ | ||
| C�� | 150L2mol•L-1KCl��Һ | D�� | 75L2mol•L-1AlCl3��Һ |
| A�� | Cl2ˮ������ɫ�Լ�ƿ�ܹⱣ�� | |
| B�� | ʢNaOH��Һ���Լ�ƿ��ĥ�ڲ����� | |
| C�� | FeSO4��Һ����ڼ����������۵��Լ�ƿ�� | |
| D�� | ������Һ�ͽ������ķ����Ƕ����ЧӦ |
�ı�ú�����÷�ʽ�ɼ��ٻ�����Ⱦ��ͨ���ɽ�ˮ����ͨ�����ȵ�̼�õ�ˮú����
��1����֪��H2��g��+1/2O2��g���TH2O��g����H1=-241.8kJ•mol-1
2C��s��+O2��g���T2CO��g����H2=-221kJ•mol-1
�ɴ˿�֪��̿��ˮ������Ӧ���Ȼ�ѧ����ʽΪC��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1
��2��ú���������в������к�����H2S����������Na2CO3��Һ���գ��÷�Ӧ�����ӷ���ʽΪCO32-+H2S=HCO3-+HS-
����֪��H2S��Ka1=1.3��10-7��Ka2=7.1��10-15��H2CO3��Ka1=4.4��10-7��Ka2=4.7��10-11��
��3���ֽ���ͬ����CO��g����H2O��g���ֱ�ͨ�˵����Ϊ2L�ĺ����ܱ������з��������Ӧ��
CO��g��+H2O��g��?CO2��g��+H2��g����H���õ���������
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ�� ����ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
��4��һ�������£�ij�ܱ��������ѽ���A��g��+B��g��?C��g��+D��g����H��0�Ļ�ѧƽ�⣬��ʱ������ͼ����ͼ1������ѡ���ж���t1ʱ�̲�ȡ�Ŀ��ܲ�������ƽ���ƶ�����ж���ȷ����A

A����Сѹǿ��ͬʱ�����¶ȣ�ƽ�������ƶ�
B������B��g��Ũ�ȣ�ͬʱ����C��g��Ũ�ȣ�ƽ�ⲻ�ƶ�
C������A��g��Ũ�ȣ�ͬʱ�����¶ȣ�ƽ�ⲻ�ƶ�
D�����������¶�ѹǿ����ͨ��ϡ�����壬ƽ�ⲻ�ƶ�
��ѹ����Ȼ����CNG���������ŵ�֮һ�����ô�������NOxת�������CO2��N2��
��CH4��g��+4NO��g��$\stackrel{����}{?}$2N2��g��+CO2��g��+2H2O��g����H1��0
��CH4��g��+2NO2��g��$\stackrel{����}{?}$��g��+CO2��g��+2H2O��g����H2��0
��5���ռ�ij����β��������NOx�ĺ���Ϊ1.12%����������������ü��齫����ȫת��Ϊ�����壬����1��104L����״���£���β����Ҫ����30g����β����V ��NO����V ��NO2��=1��1��
��6���ڲ�ͬ�����£�NO�ķֽ���ﲻͬ���ڸ�ѹ�£�NO��40���·ֽ��������ֻ������ϵ�и�������ʵ�����ʱ��仯������ͼ2��ʾ��д��Y��Z�Ļ�ѧʽ��N2O��NO2��


 һ�����Ӿ���ľ�����ͼ������������A��
һ�����Ӿ���ľ�����ͼ������������A��  ��ʾ��������B��
��ʾ��������B��  ��ʾ��
��ʾ��
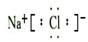 ��
��