��Ŀ����
14����ӡȾ��ҵ�У������������ƣ�Na2S2O4������ʹȾ�õIJ���ɫ����ʹ��������Ⱦɫ���ʶ��׳Ʊ��շۣ��������ˮ���������ڼ״�����ҵ�Ʊ�������ͼ��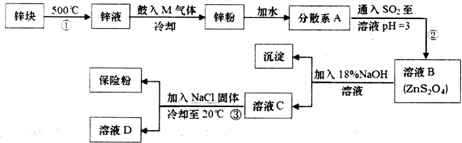
��ش��������⣺
��1�����̢ٲ������������µ������н��У��������������������Ľ��������ﻯѧʽΪAl2O3��MgO��
��2����пҺ�й���M���壬ʹҺ̬п��������ȴ�õ�����ԼΪ180��m��п�ۣ����ȱ�ʾ�����������ƽ����С�̶ȵ���ֵ����������MΪ�����Ļ������M�ĵ���ʽΪ
 ����ɢϵAΪ����Һ�����Һ�������塱������Һ������
����ɢϵAΪ����Һ�����Һ�������塱������Һ��������3�����̢ڲ����еĻ�ѧ����ʽΪZn+2SO2=ZnS2O4��
��4�����̢۲����м���NaCl���������Ϊ����Na+ Ũ�ȣ�����Na2S2O4�ᾧ���������˺��ü״�ϴ�ӡ��������Եõ������ı��շۣ�
��5���������0.04mo1•L-1AgNO3��Һ��0.02mol•L-1����Na2S2O4��Һ��ϣ�����ǡ����ȫ��Ӧ����Ӧ����Һ�����������������ɣ�д����Ӧ�����ӷ���ʽ2Ag++S2O42-+4OH-=2Ag+2SO32-+2H2O��
���� п������ڻ�����пҺ�й���M���壬ʹҺ̬п��������ȴ�õ�п�ۣ���ˮ�γɷ�ɢϵ����ͨ���������Ӧ�õ�ZnS2O4������NaOH��Һ��Ӧ�õ�������п������Na2S2O4��������NaCl����Na2S2O4���ܽ�ȣ�����Na2S2O4����ҺD�к���NaCl��
��1���������������������Ľ���������Ϊ��������
��2����п���Ʊ�п�ۣ�����п��Ӧ�ı��������������MΪ�����Ļ���������Ƕ�����̼����ȴ�õ�����ԼΪ180��m��п�ۣ���ɢϵAΪ����Һ��
��3�����̢ڲ�����Zn���������Ӧ����ZnS2O4��
��4�����̢��Ƿ����������Һ�����뷽��Ϊ���ˡ�ϴ�ӡ����Ϊ�����ܽ�µ���ʧ���ü״�ϴ�ӣ�����ǰ����NaClNa+ Ũ��������Na2S2O4�ᾧ������
��5����Ӧ����Һ�����������������ɣ����ݵ���ת���غ㣬��֪S2O42-��������SO32-��
��� �⣺��1���������������������Ľ����������ȼΪAl2O3��MgO�ȣ��ʴ�Ϊ��Al2O3��MgO��
��2����п���Ʊ�п�۵�Ŀ���ǣ�����п��Ӧ�ı�������ӿ컯ѧ��Ӧ���ʣ���пҺ�й���M���壬ʹҺ̬п��������ȴ�õ�����ԼΪ180��m��п�ۣ���������MΪ�����Ļ������M�Ļ�ѧʽΪCO2������ʽΪ ����ɢϵAΪ����Һ��
����ɢϵAΪ����Һ��
�ʴ�Ϊ�� ������Һ��
������Һ��
��3�����̢ڲ�����Zn���������Ӧ����ZnS2O4����Ӧ����ʽΪ��Zn+2SO2=ZnS2O4��
�ʴ�Ϊ��Zn+2SO2=ZnS2O4��
��4�����̢��Ƿ����������Һ�����뷽��Ϊ���ˡ�ϴ�ӡ����Ϊ�����ܽ�µ���ʧ���ü״�ϴ�ӣ�����ǰ����NaCl����Na+ Ũ�ȣ�����Na2S2O4�ᾧ������
�ʴ�Ϊ������Na+ Ũ�ȣ�����Na2S2O4�ᾧ�������״���
��5����Ӧ����Һ�����������������ɣ�AgԪ�ط�����ԭ��Ӧ��SԪ�ط���������Ӧ����SԪ�������������л��ϼ�Ϊa����VL��0.04mo1•L-1��1=VL��0.02mol•L-1��2����a-3�������a=4����S2O42-��������SO32-����Ӧ���ӷ���ʽΪ��2Ag++S2O42-+4OH-=2Ag+2SO32-+2H2O��
�ʴ�Ϊ��2Ag++S2O42-+4OH-=2Ag+2SO32-+2H2O��
���� ���⿼���������������̡�İ������ʽ����д�����ʵķ����ᴿ�ȣ�Ϊ�߿��������ͣ�ע�����Ŀ��Ϣ�Ļ�ȡ��Ǩ��Ӧ�ã��Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д� ʵ���Ҳ�����ͼ��ʾװ���Ʊ�����������ʵ�������ȡ��ʢ�б���̼������Һ���Թܣ����ظ��Թ��ڱڻ���������ɫʯ����Һ1������������ɫʯ����Һ�����ڱ���̼������Һ������������Һ��֮�䣨�������̲����Թܣ��������йظ�ʵ�������������ȷ���ǣ�������
ʵ���Ҳ�����ͼ��ʾװ���Ʊ�����������ʵ�������ȡ��ʢ�б���̼������Һ���Թܣ����ظ��Թ��ڱڻ���������ɫʯ����Һ1������������ɫʯ����Һ�����ڱ���̼������Һ������������Һ��֮�䣨�������̲����Թܣ��������йظ�ʵ�������������ȷ���ǣ�������| A�� | �Ʊ������������л���������Ҵ����� | |
| B�� | ��ʵ����Ũ����������Ǵ�����ˮ | |
| C�� | ����̼������Һ��Ҫ�����ǽ��������������ܽ�ȼ������Ҵ����к����� | |
| D�� | ʯ���Ϊ���㻷�����϶����������ϡ��� |

��ش��������⣺
��1��������пɹ۲쵽b�Թ�����ϸС������ð����д���÷�Ӧ�����ӷ���ʽ��2CH3COOH+CO32-=2CH3COO-+H2O+CO2����
��2��Aװ����ʹ�����ιܳ������������⣬��һ��Ҫ�����Ƿ�ֹ������������з���������������ʹ�õ�һ�������Ƿ�Һ©����
��3��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼA��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ�b�ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ�� ��� | �Թ�a���Լ� | �Թ�b���Լ� | ����л���ĺ��/cm |
| A | 3 mL�Ҵ���2 mL���ᡢ1mL 18mol•L-1 Ũ���� | ����Na2CO3��Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6 mL 3mol•L-1 H2SO4 | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |
�ڷ���ʵ��AC����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʣ�Ũ�������ˮ���ܹ���������������ʵ�ԭ����Ũ�����������������Ӧ�����ɵ�ˮ��������������Ũ��ʹƽ�����������������ķ����ƶ���
�ۼ���������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���Ǵ������ᡢ�Ҵ�δ����Ӧ�����뷴Ӧ��ϵ�����¶ȹ��߷���������Ӧ����
�ܷ�����������������ϴ�ӣ�Ϊ�˸�������������ѡ�õĸ����ΪB������ĸ����
A��P2O5 B����ˮNa2SO4 C����ʯ�� D��NaOH���壮
| A�� | S��ȼ����Ϊ��H=-297.23kJ/mol | |
| B�� | S��s����S��g�� �������� | |
| C�� | S��g��+O2 ��g���TSO2 ��g�� �ų�������С��297.23kJ | |
| D�� | �γ�1mol SO2��ѧ�����ͷ����������ڶ���1molS��s����1molO2 ��g���Ļ�ѧ�������յ������� |



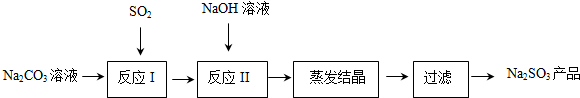
 ʵ����Ҳ������ͼ��ʾ��װ����ȡ������������������֪����������ˮ�е��ܽ�Ƚϴ�15��ʱ100gˮ�����ܽ�8.5g��
ʵ����Ҳ������ͼ��ʾ��װ����ȡ������������������֪����������ˮ�е��ܽ�Ƚϴ�15��ʱ100gˮ�����ܽ�8.5g��