��Ŀ����
19������������ӡȾ����ֽ���ڶ���ҵ�����Ź㷺��Ӧ�ã��о�С����Na2CO3��Һ����SO2�Ʊ�Na2SO3����ʵ���������£�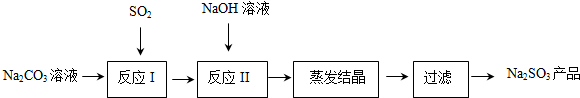
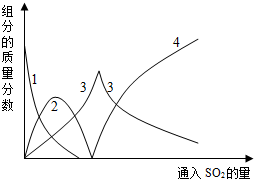
�������Ͽ�֪����̼������Һͨ���������Ĺ����У���Һ���й���ֵ����������仯��ͼ����ʾ��
��1��ͼ�е���3��ʾ�������ƣ�����2��ʾ�����ΪNaHCO3���ѧʽ��
��2��ʵ��ʱ������ӦII���м���NaOH��Һ��Ŀ����NaHSO3+NaOH=Na2SO3+H2O���û�ѧ����ʽ��ʾ��
��3�����ұ��涨��Ʒ��Na2SO3������������97.0%Ϊ�ŵ�Ʒ����93.0%Ϊһ��Ʒ��Ϊ��ȷ��ʵ�����ò�Ʒ�ĵȼ����о�С����������ַ������вⶨ��
�ٷ���I����ȡ2.570g��Ʒ��������ˮ�ܽ⣬����������˫��ˮʹNa2SO3��ȫ��������Na2SO4���ڼ��������BaCl2��Һ�����ó����������ˡ�ϴ�ӡ���������������Ϊ4.660g��ͨ������ȷ����Ʒ��Na2SO3����������98.05%��д��������̣�
�ڷ���II����ȡ1.326g��Ʒ�����100mL��Һ��ȡ25.00mL����Һ���μ�0.1250mol/L I2��Һ��ǡ��ʹNa2SO3��ȫ��������Na2SO4ʱ������I2��Һ20.00mL��ͨ������ȷ����Ʒ��Na2SO3������������д��������̣�
���ж�Na2SO3��Ʒ�ĵȼ�����˵�����ɲ�ƷΪһ��Ʒ����Ʒ�к��е������ƺ�̼���ƣ��ڷ���I�IJⶨ�У������ƺ�̼��������ʹ�òⶨֵƫ�ߣ�������II��ֱ�Ӳⶨ�������ƣ������ţ�
���� ̼������Һ��ͨ������������η����ķ�ӦΪ��2Na2CO3+SO2+H2O=2NaHCO3+Na2SO3��2NaHCO3+SO2=Na2SO3+CO2��SO2+Na2SO3=2NaHSO3����Ӧ���Ǽ�������������Һ������Ӧ��NaHSO3+NaOH=Na2SO3+H2O����Ҫ����SO2+2NaOH=Na2SO3+H2O����Ҫ�����õ�����������Һ����Ũ������ȴ�ᾧ����ϴ�ӵõ�Na2SO3��
��1�����ݷ�Ӧ���̺�ͼ�����߱仯��֪��ͼ��3��ʾ�����������ƣ�ͼ��2��ʾ����̼�����ƣ�
��2������ӦII���м���NaOH��Һ��Ŀ���Ǻ����������Ʒ�Ӧ�õ��������ƣ�
��3���ٷ���I��ȡ2.570g��Ʒ��������ˮ�ܽ⣬����������˫��ˮʹNa2SO3��ȫ��������Na2SO4���ټ��������BaCl2��Һ�����ó����������ˡ�ϴ�ӡ���������������Ϊ4.660g��Ϊ���ᱵ������������ӦΪ��Na2SO3+H2O2=Na2SO4+H2O��Na2SO4+BaCl2=2NaCl+BaSO4����������ᱵ���ʵ�������Ԫ���غ���㣻
�ڷ���II����ȡ1.326g��Ʒ�����100mL��Һ��ȡ25.00mL����Һ���μ�0.1250mol/L I2��Һ��ǡ��ʹNa2SO3��ȫ��������Na2SO4ʱ������I2��Һ20.00mL�������ķ�ӦΪNa2SO3+I2+H2O=Na2SO4+2H I�������������������õ�����������
���ж�Na2SO3��Ʒ�ĵȼ�����Ʒ��Na2SO3������������97.0%Ϊ�ŵ�Ʒ����93.0%Ϊһ��Ʒ��
��� �⣻��1�����ݷ�Ӧ���̺�ͼ�����߱仯��֪��ͼ��3��ʾ�����������ƣ���2��ʾ�����ΪNaHCO3���ʴ�Ϊ��NaHCO3��
��2������ӦII���м���NaOH��Һ��Ŀ���Ǻ����������Ʒ�Ӧ�õ��������ƣ���Ӧ�Ļ�ѧ����ʽΪ��NaHSO3+NaOH=Na2SO3+H2O��
�ʴ�Ϊ��NaHSO3+NaOH=Na2SO3+H2O��
��3���ٷ���I��Na2SO3+H2O2=Na2SO4+H2O��Na2SO4+BaCl2=2NaCl+BaSO4��
m��BaSO4��=4.660g��
n��BaSO4��=$\frac{4.660g}{233g/mol}$=0.020mol��
�������Ԫ���غ�n��Na2SO3��=n��BaSO4��=0.020mol��
m��Na2SO3��=0.020mol��126 g/mol=2.520g��
w��Na2SO3��=$\frac{2.520g}{2.570g}$��100%��98.05%��
�ʴ�Ϊ��98.05%��
�ڷ���II��Na2SO3+I2+H2O=Na2SO4+2H I
n��Na2SO3��=n��I2��=20.00mL��10-3L/mL��0.1250mol/L=0.0025mol��
m��Na2SO3��=0.0025mol��126 g/mol��$\frac{100ml}{25ml}$=1.260g��
w��Na2SO3��=$\frac{1.260g}{1.326g}$��100%��95.02%��
�ʴ�Ϊ��95.02%��
�۹��ұ��涨��Ʒ��Na2SO3������������97.0%Ϊ�ŵ�Ʒ����93.0%Ϊһ��Ʒ������I��Na2SO3������������97.0%Ϊ�ŵ�Ʒ������II�еIJ�Ʒ��Na2SO3������������93.0%Ϊһ��Ʒ�����Ƿ���I��Ʒ�к��е������ƺ�̼���Ƶ����ʣ��ڷ���I�IJⶨ�У������ƺ�̼�������ʶԲⶨ�и��ţ�������II��ֱ�Ӳⶨ�������ƣ����ŶȺ;�ȷ�ȶ�Ҫ����һЩ��
�ʴ�Ϊ����ƷΪһ��Ʒ����Ʒ�к��е������ƺ�̼���ƣ��ڷ���I�IJⶨ�У������ƺ�̼��������ʹ�òⶨֵƫ�ߣ�������II��ֱ�Ӳⶨ�������ƣ������ţ�
���� ���⿼���������Ʊ����̡���Ӧԭ������������ɵ�ʵ��ⶨ�ͺ�������Ӧ�ã���Ŀ�Ѷ��еȣ�

 �����ᣨC6H8O7����һ�ֹ�ҵԭ�ϣ��㷺Ӧ����ʳƷ��ҽҩ����ҵ����ͼ�����ֲ�ͬ�����Ʊ�һˮ�����ᾧ�壨C6H8O7•H2O���Ĺ�������ͼ���ش�������⣺
�����ᣨC6H8O7����һ�ֹ�ҵԭ�ϣ��㷺Ӧ����ʳƷ��ҽҩ����ҵ����ͼ�����ֲ�ͬ�����Ʊ�һˮ�����ᾧ�壨C6H8O7•H2O���Ĺ�������ͼ���ش�������⣺�����Ͽ�Ƭ��
��������ˮ��Һ�����ԣ�������Ʋ�����ˮ��
�ڹ�ҵ���������ᣬԭ��Ԥ�����õ��ķ���Һ��
���������ἰ�������������ʣ�
��һˮ�������ڲ�ͬ�¶��µ��ܽ�����±���
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | �� |
| �ܽ��/g | 96 | 118 | 146 | 183 | 216 | �� |
�����η��Ʊ���
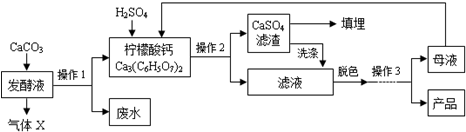
��1������X��CO2���ѧʽ����ʵ���Ҽ��������Ļ�ѧ����ʽΪCO2+Ca��OH��2�TCaCO3��+H20��
��2������1��2�������ǹ��ˣ�
��3������3��Ŀ���ǵõ�һˮ�����ᾧ�壬����˳����b��c��a��������ţ�
a������ b������Ũ�� c����ȴ�ᾧ d�������ᾧ
��4����ҵ�����У��ɼ���A������ţ�������ɫ������
A������̿ B������ C������ˮ
��5��ϴ��CaSO4������Ŀ������߲�����
��6��ĸҺ����ѭ��������һ�����е������������ᣮ
| �� | �� | |||
| ��ʼŨ�� | 5minʱŨ�� | ��ʼŨ�� | 5minʱŨ�� | |
| c��CO��/mol/L | 0.1 | 0.08 | 0.2 | x |
| c��H2O��/mol/L | 0.1 | 0.08 | 0.2 | y |
| A�� | x=y=0.16 | |
| B�� | ��Ӧ��ʼʱ�����з�Ӧ���ʱȼ� | |
| C�� | ����0��5min��ƽ����Ӧ���ʣ�v��CO��=0.004 mol/��L•min�� | |
| D�� | ƽ��ʱ������H2O��ת������50%��c��CO���Ǽ��е�2�� |
| A�� | 2NH3��g���TN2��g��+3H2��g���������ķ�Ӧ | |
| B�� | ��H����S�ֱ�ȡ������ʱ��Ӧһ�����Է����� | |
| C�� | �ڴ�����Һ�д������ַ��� | |
| D�� | ��ͬ�¶��£�pHֵ��ȵ�����ʹ��ᣬ��Ũ���Ǵ���������� |
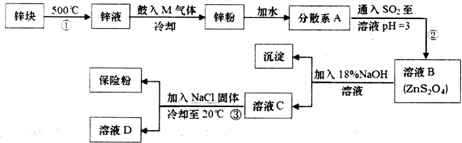
 ����ɢϵAΪ����Һ�����Һ�������塱������Һ������
����ɢϵAΪ����Һ�����Һ�������塱������Һ������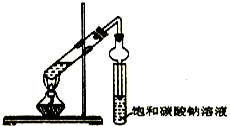 ijͬѧ��������ʾʵ��װ����ȡ�����������ش��������⣺
ijͬѧ��������ʾʵ��װ����ȡ�����������ش��������⣺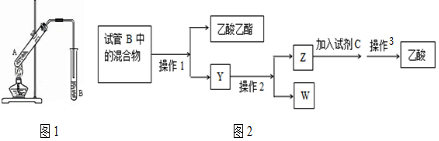
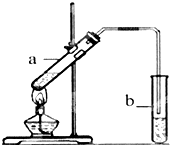 ���ѿ�������ƣ�
���ѿ�������ƣ�