��Ŀ����
18��ǰ������A��B��C��D��E5��Ԫ�ص�ԭ��������������B��Cͬ���ڣ�BA3��ʹʪ��ĺ�ɫʯ����ֽ������A��C���γ����ֻ�����1��1��2��1��A��B��D�ĵ���֮�͵���25��ED������E���ӵ�3d�ܼ����ѳ������ӣ���ش��������⣺
��1����5��Ԫ���У��縺��������O����Ԫ�ط��ţ�����̬Eԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��
��2����BA3��AD�У��е�ϸߵ���NH3���ѧʽ����ԭ���ǰ�����֮���������
��3��ABC���γ�ABC2��ABC3���ֻ������������ǿ����HNO3���ѧʽ����д��һ����BC2-��Ϊ�ĵȵ�����ķ���SO2��O3��
��4��BA4D�����к������Ӽ������Լ�����λ����������BD3�����幹��Ϊ�����Σ�����ԭ�ӵ��ӻ�����Ϊsp3��BD3����ˮ����ˮ��Һ����Ư���ԣ���ҵ�ϣ���ʯīΪ�缫�����BA4D��AD��Һ�����Ƶ�BD3����һ�ֲ���ΪH2����д���仯ѧ����ʽNH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2 ����
���� ǰ������A��B��C��D��E5��Ԫ�ص�ԭ��������������B��Cͬ���ڣ�BA3��ʹʪ��ĺ�ɫʯ����ֽ����ӦΪNH3������AΪH��BΪN��A��B��D�ĵ���֮�͵���25����DΪCl��A��C���γ����ֻ�����1��1��2��1��C��ԭ���������ڵ�������CΪO�������γ�ˮ��˫��ˮ��ED������E���ӵ�3d�ܼ����ѳ������ӣ�ECl������-1�ۣ���EΪ+1�ۣ�����EΪCu���ݴ˴��⣻
��� �⣺ǰ������A��B��C��D��E5��Ԫ�ص�ԭ��������������B��Cͬ���ڣ�BA3��ʹʪ��ĺ�ɫʯ����ֽ����ӦΪNH3������AΪH��BΪN��A��B��D�ĵ���֮�͵���25����DΪCl��A��C���γ����ֻ�����1��1��2��1��C��ԭ���������ڵ�������CΪO�������γ�ˮ��˫��ˮ��ED������E���ӵ�3d�ܼ����ѳ������ӣ�ECl������-1�ۣ���EΪ+1�ۣ�����EΪCu��
��1������Ԫ�������ɿ�֪��H��N��O��Cl��Cu��5��Ԫ���У��縺��������O����̬Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��
�ʴ�Ϊ��O��1s22s22p63s23p63d104s1��
��2����NH3��HCl�У����ڰ�����֮������������Էе�ϸߵ���NH3��
�ʴ�Ϊ��NH3��������֮���������
��3��HNO2��HNO3���ֻ������������ǿ���� HNO3����NO2-��Ϊ�ĵȵ�����ķ���ΪSO2��O3�ȣ�
�ʴ�Ϊ��HNO3��SO2��O3��
��4��NH4Cl�����к������Ӽ������Լ�����λ����������NCl3�е�ԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4����һ�Թµ��Ӷԣ����Է��ӵ� ���幹��Ϊ�����Σ�����ԭ�ӵ��ӻ�����Ϊsp3�ӻ���NCl3����ˮ����ˮ��Һ����Ư���ԣ�˵��NCl3������+1�ۣ����NH4Cl��HCl��Һ�����Ƶ�NCl3��H2����Ӧ�Ļ�ѧ����ʽΪNH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2 ����
�ʴ�Ϊ����λ���������Σ�sp3��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2 ����
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ�ƶϸ�Ԫ������Ϊ���ؼ���ע����������Ԫ�����ڱ��ṹ��Ԫ�����������ݣ�������ؿ���ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | ���ʷе㣺X��Y | B�� | �����ӵ������ԣ�W��Y | ||
| C�� | �������ˮ��������ԣ�Z��X | D�� | Y��Z�ļ����Ӿ�������ˮ�ĵ��� |
| A�� | ���ܺ���Fe2+��Fe3+ | B�� | ���ܺ���Fe2+��Cu2+ | ||
| C�� | ���ܺ���Cu2+��Fe3+ | D�� | ���ܺ��е���ͭ��Fe3+ |
| A�� | ��ʽ�ε�ˮ��Һһ�������� | |
| B�� | ̼����Һ�е�������Ũ����̼�������Ũ�ȵ�2�� | |
| C�� | �����£�pH=3����������Һ�е�������Ũ����pH=11�İ�ˮ�е�����������Ũ����� | |
| D�� | ����ʱ����pH=5��H2SO4��Һϡ��1000������c��H+����c ��SO42-��=2��1 |
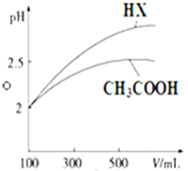 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��������Һ��a��Na2CO3 b��NaHCO3 c��NaClO d��CH3COONa�����ǵ�pH�ɴ�С���е�˳����a��c��b��d �����ţ���
��2�������£�0.1mol•L-1CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����BC��
A��c��H+�� B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$
C��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ D��c��H+��•$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
��3��CH3COOH��һԪ��HX����Һ��Ϊ100mL��pH=2����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����ͬ�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��С�ڣ�����ڡ�����С�ڡ����ڡ��� HX�ĵ���ƽ�ⳣ����
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7 mol•L-1��������λ��Ч���֣���
| A�� | �Ҵ�������͵����ʶ�����������Ӫ������ | |
| B�� | �������ֿɷ��������������溣�ʲ�Ʒ | |
| C�� | ��֬������������ˮ�����ɸ�֬������� | |
| D�� | �ϳ�������ά�����л��߷��Ӳ��� |
 ̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺
̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺