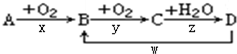
��Ŀ����
20�������仯����֮����Ի���ת�����밴��Ҫ��ش��������⣺��1��ʵ������ȡ������
��ʵ������ȡ�����ķ�Ӧԭ������������ ��������������ʵķ�Ӧ��
�ڿ��������ſ������ռ�������ԭ���ǰ������ܶ�С�ڿ������ܶȣ�
����ˮ���ն���İ���ʱ���罫����ֱ�Ӳ���ˮ�У�����������������������ԭ���ǰ���������ˮ��ʹװ���е�ѹǿС������ѹǿ��
��2���������������NO�Ļ�ѧ����ʽ��
������NԪ�صĻ�ԭ�ԣ����Ĵ�������4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
������NԪ�ص������ԣ�3Cu+8HNO3��ϡ���T3Cu��NO3��2+2NO��+4H2O��
���� ��1�����������ڼ��������·�Ӧ���ɰ�����
�����������ſ������ռ����壬��������ܶ�ӦС�ڿ����ܶȣ�
�����ݰ�����������ˮ�����ʽ��
��2���ٰ�������������һ��������ˮ����Ӧ�����ְ����Ļ�ԭ�ԣ�
��ͭ��Ũ���ᷴӦ��������ͭ��һ��������ˮ����Ӧ����������ǿ�������ԣ�
��� �⣺��1�����������ڼ��������·�Ӧ���ɰ�����ʵ�����ô������Ʊ�������
�ʴ�Ϊ���� �
�ڰ����ܶ�С�ڿ����ܶȣ�����ѡ�������ſ������ռ�������
�ʴ�Ϊ���������ܶ�С�ڿ������ܶȣ�
�۰���������ˮ��ʹװ���е�ѹǿС������ѹǿ���Ӷ���������
�ʴ�Ϊ������������ˮ��ʹװ���е�ѹǿС������ѹǿ��
��2���ٰ�������������һ��������ˮ����ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��ͭ��Ũ���ᷴӦ��������ͭ��һ��������ˮ����ѧ����ʽ��3Cu+8HNO3 ��ϡ���T3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��3Cu+8HNO3 ��ϡ���T3Cu��NO3��2+2NO��+4H2O��
���� ���⿼���˰����Ʊ����ռ������ʵļ��飬��Ϥ�����Ʊ�ԭ���������ǽ���ؼ�����Ŀ�ѶȲ���

| A�� | CH3CH3 | B�� | CH2=CH2 | C�� | CH3CH=CHCH3 | D�� | CH3CH=CH2 |
| A�� | Na2CO3�T2Na++CO32- | B�� | H2O�T2H++O2- | ||
| C�� | HNO3�TH++NO3- | D�� | K2SO4�T2K++SO42- |
| A�� | ��Һ��c��OH-����С | B�� | pH�ı仯ֵ����2 | ||
| C�� | ��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$��ֵ���� | D�� | Kw��ֵ��С |
| A�� | �Ҵ� | B�� | �ȼ��� | C�� | ������ | D�� | �������� |
 ��1����֪H-H ����Ϊ436kJ•mol-1��H-N������Ϊ391kJ•mol-1�����ݻ�ѧ����ʽ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1����N��N���ļ�����945.6 kJ/mol
��1����֪H-H ����Ϊ436kJ•mol-1��H-N������Ϊ391kJ•mol-1�����ݻ�ѧ����ʽ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1����N��N���ļ�����945.6 kJ/mol �������⣨H2O2���ǵ���ɫ����Һ�壬ˮ��ҺΪ��ɫ��Һ�壬�׳�˫��ˮ�����Կ�����Ԫ���ᣮ
�������⣨H2O2���ǵ���ɫ����Һ�壬ˮ��ҺΪ��ɫ��Һ�壬�׳�˫��ˮ�����Կ�����Ԫ���ᣮ