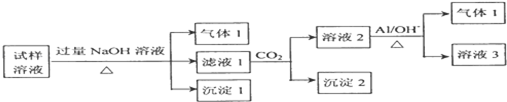
��Ŀ����
9��A��B��C��D��Ϊ��ѧ��ѧ�ij��������Ҿ�����ͬһ��Ԫ�أ�����֮���ת����ϵ��ͼ��ʾ����Ӧ���������������Ѿ���ȥ����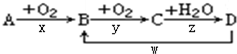
��ش��������⣺
��1����������AΪ����ɫ���嵥�ʣ�DΪǿ�ᣬ��
��A�Ļ�ѧʽ��S��
�ڷ�Ӧx��y��z��w�У�һ����������ԭ��Ӧ����x��y��
���й�B��˵������ȷ����bc��
a��ȼ�ջ�ʯȼ�ϲ������B
b����ֽ�����п���BƯ��ֽ��
c����ɫʯ����Һ��B��ˮ��Һ����
d���������Ѿ������Ӵ���B��ɱ����
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죮��
��A�������ǰ�����
�ڷ�Ӧx�Ļ�ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�۷�Ӧw�����ӷ���ʽ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
���� ��1��������AΪ����ɫ���嵥�ʣ�DΪǿ�ᣬ��A��S��B��SO2��C��SO3��D��H2SO4��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬��AΪNH3��BΪNO��CΪNO2����DΪHNO3��
��� �⣺��1��������AΪ����ɫ���嵥�ʣ�DΪǿ�ᣬ��A��S��B��SO2��C��SO3��D��H2SO4��
��A�Ļ�ѧʽ�ǣ�S���ʴ�Ϊ��S��
�ڷ�Ӧx��y��z��w�У�һ����������ԭ��Ӧ���У�x��y�Ǿ��е��ʲμӵĻ��Ϸ�Ӧ��һ������������ԭ��Ӧ���ʴ�Ϊ��x��y��
��a����ʯȼ���к�����Ԫ�أ�ȼ��ʱ�����SO2����a����
b����ֽ�����п���SO2Ư��ֽ������b��ȷ��
c��SO2��ˮ��Һ�����ԣ���ɫʯ����Һ��SO2��ˮ��Һ���죬��c��ȷ��
d���������Ѿ�������SO2��ɱ��������d����
��ѡ��bc��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬��AΪNH3��BΪNO��CΪNO2����DΪHNO3��
��A�������ǣ��������ʴ�Ϊ��������
�ڷ�Ӧx�Ļ�ѧ����ʽ�ǣ�4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�۷�Ӧw�����ӷ���ʽ�ǣ�3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
���� ���⿼��Ԫ�ػ�������ƶϣ��漰S��NԪ�ص��ʼ��仯����������ת�����Ѷ��еȣ���Ҫѧ����������Ԫ�ػ�����֪ʶ��ע����ػ���֪ʶ�Ļ��ۣ��Ѷ��еȣ�

������ͼ�ʯ�Ҷ��ǻ���
��ú��ʯ�Ͷ��ǿ�������Դ��
�����ǡ����ᱵ��ˮ�ֱ����ڷǵ���ʡ�ǿ����ʺ�������ʣ�
�ܲ���ֺ�Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
�����ᡢ��������ƺ���ʯ�ҷֱ������ᡢ��κ������
�����������ǽ��壮
����˵����ȷ���ǣ�������
| A�� | �٢ڢܢ� | B�� | �٢ݢ� | C�� | �٢ۢܢ� | D�� | �ڢۢܢ� |
| A�� | Mg | B�� | Fe | C�� | Cu | D�� | Na |
| A | B | C | D | |
| �������� | ���� | С�մ� | �������� | ������ |
| ��; | ��Ư�� | ����θ����� | �ư뵼����� | ������ɫͿ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 2015��˹̹����ѧ�о���Ա���Ƴ�һ�ֿ���һ��������ɳ�ŵ�ij������������ӵ�أ��ڲ���AlCl4-���л������ӹ��ɵ������Һ����ŵ繤��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������
2015��˹̹����ѧ�о���Ա���Ƴ�һ�ֿ���һ��������ɳ�ŵ�ij������������ӵ�أ��ڲ���AlCl4-���л������ӹ��ɵ������Һ����ŵ繤��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �ŵ�ʱ����Ϊ������ʯīΪ���� | |
| B�� | �ŵ�ʱ���л������������缫�����ƶ� | |
| C�� | �ŵ�ʱ�ĸ�����ӦΪ��Al-3e-+7AlCl4-�T4Al2Cl7- | |
| D�� | ���ʱ��������ӦΪ��Cn+AlCl4--e-�TCnAlCl4 |
| A�� | ��ѿ��ˮ��IJ���ֻ�������� | |
| B�� | ���ӿ����ںϳɸ߷��ӻ����� | |
| C�� | ��֬ˮ�������ȡ��֬���� | |
| D�� | ������������Ǧ������ij�������������ˮ |
 ��������20mL 0.1mol/LCH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���д�����ǣ�������
��������20mL 0.1mol/LCH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���д�����ǣ�������| A�� | a���ʾ����Һ��c��CH3COO-����С��10-3mol/L | |
| B�� | b���ʾ����Һ��c��CH3COO-����c��Na+�� | |
| C�� | c���ʾCH3COOH��NaOHǡ����ȫ�к� | |
| D�� | �ζ���������Һ�е�n ��CH3COO-��+n��CH3COOH���ĺͲ��� |