��Ŀ����
2��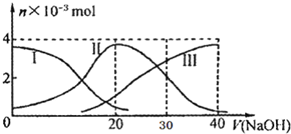 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�жϣ�����˵������ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-��������ͼʾ�жϣ�����˵������ȷ���ǣ�������| A�� | H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A�TH++HA-��HA-�TH++A2- | |
| B�� | ��V��NaOH��=20mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� | |
| C�� | �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮС | |
| D�� | ��V��NaOH��=30mLʱ����Һ�д������¹�ϵ��2c��H+��+c��HA-��+2c��H2A��=c��A2-��+2 c��OH-�� |
���� A����V��NaOH��=30mLʱ����Һ��H2A�����H2A��������ʣ�H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A?H++HA-��HA-?H++A2-��
B������ͼ��֪����V��NaOH��=20ʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA������Ϊ������Һ�����ԣ�
C����������ˮ���룬�����������ӵ���ˮ��ٽ�ˮ���룻
D����V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����Һ��ҪΪ����������NaHA��Na2A�Ļ����Һ�����ݵ���غ�������غ㣮
��� �⣺A��H2A��������ʣ�H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A?H++HA-��HA-?H++A2-����A����
B����V��NaOH��=20 mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA��HA-�������ˮ�⣬��Һ�����ԣ���c��Na+����c��HA-����c��H+����c��A2-����c��OH-������B��ȷ��
C����ͼʾ��ϵ֪��c��A2-����c��H2A����˵���������ˮ��̶ȣ���Һ�����ԣ�ˮ�ĵ����ܵ������ƣ���C����
D����V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����Һ��ҪΪ����������NaHA��Na2A�Ļ����Һ�����ݵ���غ�ã�c��Na+��+c��H+��=c��HA-��+2c��A2-��+c��OH-���٣������غ��֪��3c��HA-��+3c��A2-��+3c��H2A��=2c��Na+���ڣ��١�2+�ڵã�2c��H+��+c��HA-��+3c��H2A���Tc��A2-��+2c��OH-������D����
��ѡB��
���� ���⿼���������Һ�����жϣ�Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ����������ѧ���ķ��������Ŀ��飬��ȷͼ���������ʱ��Һ�е������ǽ����Ĺؼ���ץסͼ����з������ɣ�

 ��������ϵ�д�
��������ϵ�д� �����Ϊ0.5L��AlCl3��Һ������ijŨ�ȵ�NaOH��Һ���õ��ij�����NaOH��Һ����ı仯��ͼ��ʾ�����н����ȷ���ǣ�������
�����Ϊ0.5L��AlCl3��Һ������ijŨ�ȵ�NaOH��Һ���õ��ij�����NaOH��Һ����ı仯��ͼ��ʾ�����н����ȷ���ǣ�������| A�� | ��Ӧ�����У��������ʱ������Ϊ7.8g | |
| B�� | AlCl3��Һ��Ũ��Ϊ2.0��mol•L-1 | |
| C�� | �õ�39g����ʱ�����ĵ�NaOH��Һ���Ϊ1.5L��3.5L | |
| D�� | ��V��NaOH��=4Lʱ���õ�����Һ��Na+��Cl-Ũ����� |
| A�� | Y���ʲ�����X���⻯�ﷴӦ | |
| B�� | Y���⻯�������ӻ����� | |
| C�� | ��ҵ��ұ��Zͨ���õ���������Ȼ���ķ��� | |
| D�� | W���ʼ������ᷴӦ��������Ӧ�������������� |
 ��ͼ��ʾ��ʵ�飬�����ձ�������KMn04��Һ��ɫ�������ձ��е���Һ���ɺ�������KSCN��FeS04��Һ����Һ��Ѫ��ɫ���ж�����˵���в���ȷ���ǣ�������
��ͼ��ʾ��ʵ�飬�����ձ�������KMn04��Һ��ɫ�������ձ��е���Һ���ɺ�������KSCN��FeS04��Һ����Һ��Ѫ��ɫ���ж�����˵���в���ȷ���ǣ�������| A�� | ��������Hzȼ�������˼Ⱦ����������־��л�ԭ�Ե����� | |
| B�� | ��������H2ȼ�յIJ����п��ܺ���һ������H202 | |
| C�� | ���ձ�����Һ����KI������ҺҲ����֤��������л�ԭ�� | |
| D�� | ����FeSO4��Һ�м���˫��ˮ�����ӷ�ӦΪ��2Fe2++H2O2+2H+=2Fe3++2H20 |
| A�� | Na2CO3��Һ����ʢװ�ڲ��������Լ�ƿ�� | |
| B�� | ����ʳ��ˮʹ�����Ի�ɫ | |
| C�� | FeCl3��Һ�������ɵõ�Fe2O3 | |
| D�� | 0.1mol/LCuCl2��Һ�У�c��Cu2+����0.1mol/L |