��Ŀ����
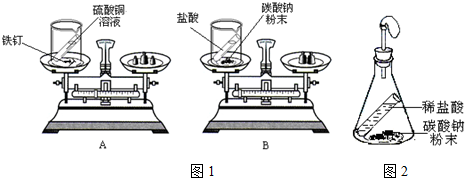
7��ijͬѧ����ȡ��Ҷ����ǩ������Ҫ50g��������Ϊ10%������������Һ����ش��������⣺��1�������������ƹ�������
�����ȡ��������5g��
����������ƽ�����������������ʱ������������ƽ��ָ��ƫ�����̣�ӦD��
A���������롡��B���������롡��������C�����������������ƹ��塡������D�����������������ƹ���
������ͬѧ��ȡˮʱ�����Ӷ������������Ƶ�����������Һ����������ƫС���ƫ����ƫС�����䡱����
��2������25%������������Һ����
����Ҫ25%������������Һ20g��
�ڸ���Һ���ƹ����У����õ���Ͳ���������ͽ�ͷ�ι��⣬����Ҫ���������ձ���
��3��20��ʱ�����ⶨNaOH��Һ��pH�����Ƚ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pHƫС���ƫ����ƫС������Ӱ�족����
���� ��1���ٸ�����������=��Һ���������������������ܼ�����=��Һ����-�����������㣻
�ڳ����������������ʱ������������ƽ��ָ��ƫ�����̣����ж�ҩƷ����ƫ�ݴ�ѡ������������еIJ�����
��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����ӻ��Ӷ�����ɶ�����ȷ���
��2��
�ٸ���Ũ��Һϡ��Ϊϡ��Һ�����ʵ�����������
�ڸ�����Һ���ƹ��������õ��������
��3�����ݽ�pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pHֵ����ʹ������������Һ�ļ��Ա������н��
��� �⣺��1������Ҫ�������Ƶ�����Ϊ��50g��10%=5g��
�ڸ���ͼʾ����ƽ��ʹ�÷���������������ƽ��ָ��ƫ������ʱ����ȡ������������ƫ����Ҫ�����������ƣ�����ӦѡD��
��ʹ����Ͳ����ʱ��Ҫʹ������Һ�尼Һ�����ʹ�����ˮƽ�����Ӷ���С����ȡˮ�����ƫ���������Ƶ�����������Һ����������ƫС��
��2��
��Ũ��Һϡ��Ϊϡ��Һ�����ʵ��������䣬����Ҫ25%������������Һ������Ϊx������5g=x��25%�����x=20g��
�ڸ���Һ���ƹ����У����õ���Ͳ���������ͽ�ͷ�ι��⣬����Ҫ���������ձ���
��3����pH��ֽ������ˮ��ʪ���ٽ��вⶨ����������Һ��pHֵ����ʹ������������Һ�ļ��Ա������������������Һ��pHƫС�����ƫС��
�ʴ�Ϊ����1����5����D����ƫС����2����20�����ձ�����3��ƫС��
���� ������Һ���ֳ����������������ʼ�ˮ�ܽ⣬���Ʋ������-����-�ܽ⣻Һ���ˮϡ�ͣ����Ʋ������-��ȡ-�ܽ⣮

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�| A�� | �ྻ�Ŀ��� | B�� | Һ�� | C�� | ��ˮ | D�� | ���� |
| A�� | �������MgCO3��NaHCO3��������һ����1��1 | |
| B�� | ����ϡ���������������������Ϊ5% | |
| C�� | �������MgCO3��NaHCO3�������κα�����ϣ����Ĵ�ϡ�������������Ϊ100g | |
| D�� | �������MgCO3��NaHCO3�������κα�����ϣ����ɶ�����̼����������Ϊ4.4g |
| A�� | �� | B�� | �� | C�� | �� | D�� | п |
| ѡ�� | ʵ��Ŀ�� | �����Լ��� |
| A | ������ë����� | ��ȼ������ζ |
| B | �������������̼ | ����ȼ��ľ�����۲����� |
| C | ��ȥ������̼��������һ����̼ | ͨ�����������ȵ�ͭ�� |
| D | ���鵪���Ƿ���NH${\;}_{4}^{+}$ | ����ʯ����ĥ����ζ |
| A�� | NH4Cl | B�� | K2CO3 | C�� | KNO3 | D�� | Ca��H2PO4��2 |