��Ŀ����
2��ʵ������һƿ���ڱ�¶�ڿ����е��������ƹ�����Ʒ��ij��ȤС���ͬѧ�Ը���Ʒ�ijɷּ�����������̽����
��ʵ��̽��1��
��1��Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�森
| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ���ɫ | ��Ʒ�к����������� |
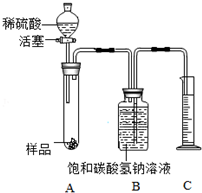
�ٰ�ͼ���Ӻ�װ�ò����װ�������ԣ�
����������ƽȷ��ȡ6g����Ʒ������A���Թ��ڣ���B�м���ƿ�е��뱥��̼��������Һ��ƿ������
�����Һ©���м���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ�����Һ110mL��
��2��д��A�з�����Ӧ��һ����ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3��ʵ����ȡ����Ʒ���������˹��࣬�����Ʒ�������࣬��ɵĺ������Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4����֪��ʵ�������£�������̼���ܶ�Ϊ2g•L-1��������CO2������Ϊ0.22g����Ʒ��̼���Ƶ���������Ϊ88.3%��
��5��ʵ������У�������ȷ��װ�����������ã�Na2CO3���������Խ�ƫ���ƫ��ƫС�����������Ǽ���������Һ�����Ҳ�����������̼����������̼����ƫ�࣬����̼���Ƶ���������ƫ��
��ʵ��̽��3��Ϊ�˵õ��ϴ������������ƹ��壬����ͬѧ�������ͼ��ʾ��ʵ�����̣�
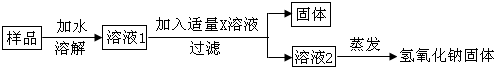
��6����������X��Һ��Ca��OH��2��Һ��
��7�����������������ƹ�����������ڱ�����Ʒ���������Ƶ�������ѡ����ڡ��������ڡ���С�ڡ�����
���� ��1������̼���ơ�������������Һ�ж��Լ��ԣ��������������÷�̪����Ҫ�Ƚ�̼���Ƽ��鲢��ȥ�������������������ӽ��з�����
��2�������������̼���ơ��������Ʒ�Ӧ���з�����
��3�������������ƺ����ᷢ�����кͷ�Ӧ��ų�������ʹ����������ͽ��з�����
��4�����ݶ�����̼���ܶȺ���������������̼��������Ȼ���ϻ�ѧ����ʽ�����̼���Ƶ�������
��5�����ݼ����ϡ������ų�һ���������������ʹ����Ķ�����̼���ƫ��̼���Ƶ���������Ҳ��ƫ����з�����
��6�������������Ʊ�������̼���ƣ���ȥ̼���ƿ��Լ�������������������Һ��
��7������̼���ƺ��������Ʒ�Ӧ������̼��Ƴ������������ƽ��з�����
��� �⣺��1��̼���ơ�������������Һ�ж��Լ��ԣ��������������÷�̪����Ҫ�Ƚ�̼���Ƽ��鲢��ȥ�������������������ӣ�����
| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ��ɺ�ɫ | ��Ʒ�к����������� |
��3���������ƺ����ᷢ�����кͷ�Ӧ��ų�������ʹ����������ͣ�������Ʒ�������࣬��ɵĺ���ǣ���Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4�����ɶ�����̼������Ϊ��$\frac{110mL}{1000}$��2g•L-1=0.22g��
��μӷ�Ӧ��̼���Ƶ�����Ϊx��
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
106 44
x 0.22g
$\frac{106}{x}$=$\frac{44}{0.22g}$
x=0.53g
������Ʒ��̼���Ƶ���������Ϊ��$\frac{0.53g}{6g}$��100%��8.83%��
��5�������ϡ������ų�һ���������������ʹ����Ķ�����̼���ƫ��̼���Ƶ���������Ҳ��ƫ��
��6���������Ʊ�������̼���ƣ���ȥ̼���ƿ��Լ�������������������Һ������X������������Һ��
��7��̼���ƺ��������Ʒ�Ӧ������̼��Ƴ������������ƣ��������������������ƹ�����������ڱ�����Ʒ���������Ƶ�������
�ʴ�Ϊ����1��
| ʵ����� | ʵ������ | ʵ����� |
| �������Ȼ�����Һ | ||
| ��̪��Һ�� | ��Һ��ɺ�ɫ |
��3����Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4��0.22g��8.83%��
��5��ƫ����������Һ�����Ҳ�����������̼����������̼����ƫ�࣬����̼���Ƶ���������ƫ��
��6������������Һ��
��7�����ڣ�
���� �ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ��������������ʾ���н��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
12������ʵ������У���ȷ���ǣ�������
| A�� | ����ϡ����ʱ����������Ͳ�м���һ������Ũ���ᣬ������ע��ˮ�����Ͻ��� | |
| B�� | ����ʵ���У����������ڵ�Һ����ȫ���ɺ�����ֹͣ���� | |
| C�� | ��©������Һ��ʱ��Һ���Ը�����ֽ��Ե | |
| D�� | ���װ��������ʱ��Ӧ�Ȱѵ���һ�˷���ˮ�У���˫�ֽ����Թ� |
17����ʯȼ��ȼ�ղ����Ĵ����Ķ�����̼�������ж�����̼�������ᵼ������ЧӦ�Ȼ������⣺
��1��CH4��ȫȼ�յĻ�ѧ����ʽ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
��2������1��CH4��ȫȼ�ղ�����CO2����m=2.75g��
��3����ú��ȣ�ʹ����Ȼ�������ڼ�������ЧӦ������±��з�������˵������������ͬ�����ļ����̼��ȫȼ�գ�����ų���������������Ķ�����̼��
��1��CH4��ȫȼ�յĻ�ѧ����ʽ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
��2������1��CH4��ȫȼ�ղ�����CO2����m=2.75g��
��3����ú��ȣ�ʹ����Ȼ�������ڼ�������ЧӦ������±��з�������˵������������ͬ�����ļ����̼��ȫȼ�գ�����ų���������������Ķ�����̼��
| 1g������ȫȼ�շų���CO2���� | 1g������ȫȼ�շų������� | |
| CH4 | m | 56KJ |
| C | 3.67g | 32kJ |
7��Сǿͬѧ�Ļ�ѧ�ʼDZ��������¼�¼������Ϊ����ȷ���ǣ�������
| A�� | ��ѧ��Ӧǰ��ԭ�ӵĸ������������ | |
| B�� | �����ڻ�ѧ��Ӧǰ�����������ͻ�ѧ����û�з����仯 | |
| C�� | �������ڻ�����䣬�����������Ŀ�ȼ�� | |
| D�� | ϴ�Ӽ�����ȥ���ۣ�����Ϊ�������黯���� |


