��Ŀ����
13����ʽ̼���ι㷺Ӧ���ڹ�ũҵ��������ij��ʽ̼������ƷM��OH��2•MCO3 ����M��ʾij����Ԫ�أ���ʽ̼����M��OH��2•MCO3�ڼ��������¿ɷֽ�����M�������ˮ�Ͷ�����̼��Ϊȷ������M�����ԭ��������ij�о�С���������ʵ��װ�ü����裨������ֲ�������ܽ�CO2������Ӱ�죩��

��1���뽫����ʵ�鲽�貹��������
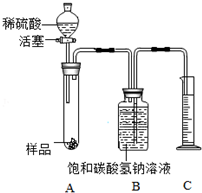
������װ�ò����װ�õ������ԣ� ��ȷ������Ʒ��������
��װҩƷ����������װ������Һ����ƽ�������� �ܼ�������Ʒ�������ټ��٣�
����ȴ�����£� ��������װ������Һ����ƽ����������ʽ����
��2��װ��B�������Ǹ���CO2���壬D������ֲ���͵������Ƿ�ֹCO2����ˮ��C��ʢ�ŵ�ҩƷΪc������ţ���
a����ʯ�� b��Ũ���� c����ɫ��ˮ����ͭ
��3����������������ȷ�������������ؿ�ʹM���ԭ�������IJⶨ���ƫ�����a������ĸ��ţ���
a����Ӧǰ������ˮ�浽0�̶ȴ�����Ӧ�����Ӷ���
b����Ʒδ�ֽ���ȫ��ֹͣʵ��
c����������δ��ֲ����
��ʵ���������õ��˼�ʽ̼��ͭ�����ɱ�ʾΪCu��OH��2•xCuCO3������x��ʾ������
ij�о�С����ʹ������װ�òⶨx��ֵ�� ���3���������±���ʾ����֪���³�ѹ��CO2���ܶ�Ϊ1.964g/L��ȡ3�����ݵ�ƽ��ֵ�õ�CO2������Ϊ0.044g��
�־ݴ˼���x��ֵ����д������x�Ĺ��̣�������������λС����
| ��� | ��Ʒ���� | CO2����� |
| ��1�� | 0.21g | 22.39mL |
| ��2�� | 0.21g | 22.41mL |
| ��3�� | 0.21g | 22.40mL |
���� ��Ҫ��Ϥ�������������ơ���;��ʹ�÷�����
Ũ���������ˮ�ԣ���������������������������̼������ĸ������
������̼�ܹ�����ˮ��������ֲ���ͣ�
��ɫ��ˮ����ͭ�ܺ�ˮ��Ӧ������ɫ��ˮ����ͭ��
���ݶ�����̼��������ܶȿ��Լ��������̼�������������ṩ�����ݿ��Լ���x��ֵ��
��� �⣺��1��������װ�ò����װ�õ������ԣ�
��ȷ������Ʒ��������
��װҩƷ����������װ������Һ����ƽ��������
�ܼ�������Ʒ�������ټ��٣�
����ȴ�����£�
��������װ������Һ����ƽ����������ʽ���㣮
������װ�õ������ԣ�
��2��װ��B�������Ǹ���CO2���壻
D������ֲ���͵������Ƿ�ֹCO2����ˮ��
C��Ӧ�÷Ű�ɫ��ˮ����ͭ���Լ��������̼�е�ˮ�����Ƿ�Ũ������ȫ���գ�
�������CO2���壻��ֹCO2����ˮ��c��
��3��a����Ӧǰ������ˮ�浽0�̶ȴ�����Ӧ�����Ӷ����ᵼ�¶���ƫС���Ӷ����¼����M�����ԭ������ƫ��
b����Ʒδ�ֽ���ȫ��ֹͣʵ�飬��Ӱ������M�����ԭ��������
c����������δ��ֲ����ʱ���ᵼ��һ���ֶ�����̼����ˮ���Ӷ����¼���Ķ�����̼����ƫС����һ�����¼����M�����ԭ������ƫС��
���a��
������̼��ƽ�����Ϊ����22.39mL+22.41mL+22.40mL����3=22.4mL��
�ⶨ���ɵĶ�����̼������Ϊ��22.4mL��10-3��1.964g/L=0.044g��
���0.044��
Cu��OH��2•xCuCO3$\frac{\underline{\;\;��\;\;}}{\;}$��1+x��CuO+H2O+xCO2��
98+124x���������������������� ��44x
0.21g 0.044g
$\frac{98+124x}{0.21g}$=$\frac{44x}{0.044g}$��
x�T1.14��
��x��ֵ��1.14��
���� �������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�| A�� | ��������ά����ҩƬ�������彡�� | B�� | ������������Ҫ�Ĺ������� | ||
| C�� | ����������Ҫ��Ӫ������ | D�� | �������л��� |
 �ס������ֹ������ʵ��ܽ��������ͼ��ʾ�������й������д�����ǣ�������
�ס������ֹ������ʵ��ܽ��������ͼ��ʾ�������й������д�����ǣ�������| A�� | t2��ʱ���ܽ�ȱ��ҵĴ� | |
| B�� | t1��ʱ�����ҵ��ܽ����� | |
| C�� | �����¶ȿɽ��ı�����Һ��Ϊ��������Һ | |
| D�� | ���ס��ҵı�����Һ��t2�潵��t1�棬�����ļ������� |

��ʵ��̽��1��
��1��Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�森
| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ���ɫ | ��Ʒ�к����������� |
�ٰ�ͼ���Ӻ�װ�ò����װ�������ԣ�
����������ƽȷ��ȡ6g����Ʒ������A���Թ��ڣ���B�м���ƿ�е��뱥��̼��������Һ��ƿ������
�����Һ©���м���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ�����Һ110mL��
��2��д��A�з�����Ӧ��һ����ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3��ʵ����ȡ����Ʒ���������˹��࣬�����Ʒ�������࣬��ɵĺ������Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4����֪��ʵ�������£�������̼���ܶ�Ϊ2g•L-1��������CO2������Ϊ0.22g����Ʒ��̼���Ƶ���������Ϊ88.3%��
��5��ʵ������У�������ȷ��װ�����������ã�Na2CO3���������Խ�ƫ���ƫ��ƫС�����������Ǽ���������Һ�����Ҳ�����������̼����������̼����ƫ�࣬����̼���Ƶ���������ƫ��
��ʵ��̽��3��Ϊ�˵õ��ϴ������������ƹ��壬����ͬѧ�������ͼ��ʾ��ʵ�����̣�
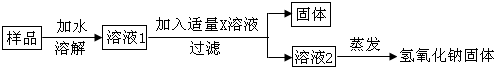
��6����������X��Һ��Ca��OH��2��Һ��
��7�����������������ƹ�����������ڱ�����Ʒ���������Ƶ�������ѡ����ڡ��������ڡ���С�ڡ�����
 �����ʷ��������������ڻ�ѧ֪ʶ��ϵͳ������ͼ��ijͬѧ�Գ���������ķ���������
�����ʷ��������������ڻ�ѧ֪ʶ��ϵͳ������ͼ��ijͬѧ�Գ���������ķ���������
