��Ŀ����
17����ʯȼ��ȼ�ղ����Ĵ����Ķ�����̼�������ж�����̼�������ᵼ������ЧӦ�Ȼ������⣺��1��CH4��ȫȼ�յĻ�ѧ����ʽ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
��2������1��CH4��ȫȼ�ղ�����CO2����m=2.75g��
��3����ú��ȣ�ʹ����Ȼ�������ڼ�������ЧӦ������±��з�������˵������������ͬ�����ļ����̼��ȫȼ�գ�����ų���������������Ķ�����̼��
| 1g������ȫȼ�շų���CO2���� | 1g������ȫȼ�շų������� | |
| CH4 | m | 56KJ |
| C | 3.67g | 32kJ |
���� ���ݼ���ȼ�����ɶ�����̼��ˮ�����ݷ���ʽ�ҳ������������̼֮���������ϵ���ɼ���������Ϳ����������������̼�����������ɱ����ṩ����Ϣ���з����ȽϿ��Եó�����Ȼ����ȼ�ϵ��ŵ㣮
��� �⣺
��1��������ȫȼ�յĻ�ѧ����ʽΪ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O�����CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��
��2����1gCH4��ȫȼ�ղ���CO2������m����
CH4 +2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
16 44
1g m
$\frac{16}{1g}=\frac{44}{m}$
m=2.75g
��3����ͼ��֪��Ȼ��ȼ�պ�����������࣬�ŷŵĶ�����̼�٣�
�𰸣�
��1��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
��2��2.75g
��3����ͬ�����ļ����̼��ȫȼ�գ�����ų���������������Ķ�����̼��
���� �˽���������ú��˵��һ�ֽ�����ȼ�ϣ����ϱ�����������ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
12����������C��H��O��Ca����Ԫ�����������ʲ�ȫ����
| ��ѧʽ | ���ʷ��� | һ�ֻ�ѧ���� | һ����; |
| ���磺CaCO3 | �� | �����ᷴӦ | ��ȡ������̼ |
| ��1��Ca��OH��2 | �� | �����ᷴӦ | ��2�������������� |
| ��3��CaO | ��4�������� | ����ˮ��Ӧ | ����ʳƷ����� |
2��ʵ������һƿ���ڱ�¶�ڿ����е��������ƹ�����Ʒ��ij��ȤС���ͬѧ�Ը���Ʒ�ijɷּ�����������̽����

��ʵ��̽��1��
��1��Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�森
��ʵ��̽��2��Ϊ�˲ⶨ��Ʒ��Na2CO3����������������ͬѧ�����ͼװ�ã�����̨��ȥ�������в������ʵ�飮
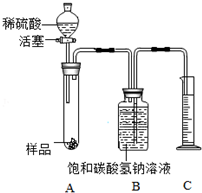
�ٰ�ͼ���Ӻ�װ�ò����װ�������ԣ�
����������ƽȷ��ȡ6g����Ʒ������A���Թ��ڣ���B�м���ƿ�е��뱥��̼��������Һ��ƿ������
�����Һ©���м���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ�����Һ110mL��
��2��д��A�з�����Ӧ��һ����ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3��ʵ����ȡ����Ʒ���������˹��࣬�����Ʒ�������࣬��ɵĺ������Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4����֪��ʵ�������£�������̼���ܶ�Ϊ2g•L-1��������CO2������Ϊ0.22g����Ʒ��̼���Ƶ���������Ϊ88.3%��
��5��ʵ������У�������ȷ��װ�����������ã�Na2CO3���������Խ�ƫ���ƫ��ƫС�����������Ǽ���������Һ�����Ҳ�����������̼����������̼����ƫ�࣬����̼���Ƶ���������ƫ��
��ʵ��̽��3��Ϊ�˵õ��ϴ������������ƹ��壬����ͬѧ�������ͼ��ʾ��ʵ�����̣�
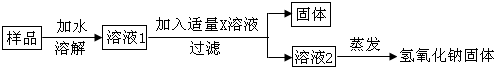
��6����������X��Һ��Ca��OH��2��Һ��
��7�����������������ƹ�����������ڱ�����Ʒ���������Ƶ�������ѡ����ڡ��������ڡ���С�ڡ�����

��ʵ��̽��1��
��1��Ϊȷ������Ʒ�ijɷ֣�С�����������ʵ�鷽��������һ���������ʵ�鱨�森
| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ���ɫ | ��Ʒ�к����������� |

�ٰ�ͼ���Ӻ�װ�ò����װ�������ԣ�
����������ƽȷ��ȡ6g����Ʒ������A���Թ��ڣ���B�м���ƿ�е��뱥��̼��������Һ��ƿ������
�����Һ©���м���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ�����Һ110mL��
��2��д��A�з�����Ӧ��һ����ѧ����ʽΪ2NaOH+H2SO4=Na2SO4+2H2O��
��3��ʵ����ȡ����Ʒ���������˹��࣬�����Ʒ�������࣬��ɵĺ������Ʒ���࣬�кͷ�Ӧ����̫��ʹ���������ͣ��������ƫ��
��4����֪��ʵ�������£�������̼���ܶ�Ϊ2g•L-1��������CO2������Ϊ0.22g����Ʒ��̼���Ƶ���������Ϊ88.3%��
��5��ʵ������У�������ȷ��װ�����������ã�Na2CO3���������Խ�ƫ���ƫ��ƫС�����������Ǽ���������Һ�����Ҳ�����������̼����������̼����ƫ�࣬����̼���Ƶ���������ƫ��
��ʵ��̽��3��Ϊ�˵õ��ϴ������������ƹ��壬����ͬѧ�������ͼ��ʾ��ʵ�����̣�

��6����������X��Һ��Ca��OH��2��Һ��
��7�����������������ƹ�����������ڱ�����Ʒ���������Ƶ�������ѡ����ڡ��������ڡ���С�ڡ�����
9����������C3H8O3���������ͣ��ǻ�ױƷ�еı�ʪ�������п���������ǣ�������
| A�� | ������Ͽ�����������̼���⡢������Ԫ����� | |
| B�� | �ӽṹ�Ͽ�����������3��̼ԭ�ӡ�8����ԭ�Ӻ�3����ԭ�ӹ��� | |
| C�� | ����������Ͽ����������Ǻ��������� | |
| D�� | �ӱ仯�Ͽ�����������ȫȼ������ˮ�Ͷ�����̼ |
6������������ȷ���ǣ�������
| A�� | ͨ��ʳƷ���Ӽ���ҩ�����ֲ���ʳ������ȡ�����Ӫ��Ԫ�� | |
| B�� | �����ּ�����Ȼ��й©��Ӧ�����رյ�� | |
| C�� | ���еĴ��ס���۵���ʳӦ����ܱմ��� | |
| D�� | ������Ĺ��ܿ���ֺ�ɫ��Ӧ������������ȼ���Ľ����� |
7��ijͬѧ�ڼҲ������۷����ˣ��۷�Ĵ�Һ�����ԣ�Ϊ�˼���ʹ���������˴�ͿĨ�������ǣ�������
| A�� | ţ�̣�pH��6�� | B�� | ƻ��֭��pH��3�� | C�� | ���ࣨpH��9�� | D�� | ��Ȫˮ��pH��7�� |
 �����ʷ��������������ڻ�ѧ֪ʶ��ϵͳ������ͼ��ijͬѧ�Գ���������ķ���������
�����ʷ��������������ڻ�ѧ֪ʶ��ϵͳ������ͼ��ijͬѧ�Գ���������ķ���������