��Ŀ����
18�� ��һ�û�ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1%��ϡ�������ⶨ�����ʵ����������������Ǽ���ͬѧ�����뼰������
��һ�û�ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1%��ϡ�������ⶨ�����ʵ����������������Ǽ���ͬѧ�����뼰��������1������ͬѧ�������ǣ����ձ��м���һ����������������Һ���õι���������1%��ϡ��
�ᣬ�����Ͻ��裬ͨ����pH��ֽ��βⶨ��ҺpH�İ취���ﵽʵ��Ŀ�ģ�
������ʹ��pH��ֽ�ķ�����ȷ����B������ĸ����
A����pH��ֱֽ�Ӳ������Һ��
B����pH��ֽ���ڸɾ��İ״ɰ��ϣ��ò�����պȡ����Һ����pH��ֽ��
C����pH��ֽ��ʪ����ڲ���Ƭ�ϣ��ò�����պȡ����Һ����pH��ֽ��
��������pH��ֽ���βⶨ���Ϸ�������������ֵ������ȷ������ʦָ���£�����ͬѧȡ��5g����������Һ���������ֻ�ʵ�飬�ɼ��������������ʵ���������ҺpH�ı仯ͼ��ʾΪͼ����
��ش�ͼ��a���ʾ�ĺ�����ǡ���кͣ�b����Һ�к��е���������Na+��H+ �������ӷ��ţ�������ͬѧҪ�����������ƿ����������Һ�����ʵ���������������Ҫ�õ����������⣬����Ϊ����Ҫ��������ϡ������ܶȣ�
��2������ͬѧ�ڵμ�ϡ����һ��ʱ�������Һ�����������ݲ�������һ���쳣�����������ǵ�̽��������ͨ��ʵ��֤���������õ���ƿ����������Һ�Ѿ����ֱ��ʣ�
���û�ѧ����ʽ��ʾ����ʵ�ԭ��CO2+2NaOH=Na2CO3+H2O��
��Ҫ��ȥ��Һ�б������ɵ����ʣ�����ʵ�鷽�����е��ǣ�CD������ĸ����
A��ȡ���ʺ����Һ���������Ȼ�����Һ������
B��ȡ���ʺ����Һ���������������Һ������
C��ȡ���ʺ����Һ������������ʯ��ˮ������
D��ȡ���ʺ����Һ������������������Һ������
E��ȡ���ʺ����Һ�����������������ٲ������ݣ�
���� ��1���ٸ���pH��ֽʹ�÷��������жϣ�
�ڸ�����ҺpH�ı仯ͼ������壬����a�ĺ��塢b����Һ�е������ӣ��������������ܶȿ���������������
��2���������������������̼�ķ�Ӧд���������Ʊ��ʵķ���ʽ������̼���Ƶ����ʷ�����Ƴ�ȥ̼���Ƶ�ʵ�鷽����
��� �⣺��1����A����pH��ֱֽ�Ӳ������Һ�У�����Ⱦ�Լ�����������
B����pH��ֽ���ڸɾ��İ״ɰ��ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ�������ȷ��
C����pH��ֽ��ʪ��ü�Һ��pHƫС����������
������ҺpH�ı仯ͼ���֪����a��ʱ����Һ��pH����7��˵�����������ƺ�����ǡ���кͣ���b��ʱ����Һ�����ԣ���������ȫ�������ᷴӦ�������Ȼ��ƣ���Һ�л���ʣ������ᣮ������Һ�е��������ǣ�Na+��H+��Ҫ������������Һ������������������֪����������������е�������֪������Ҫ��������ϡ������ܶȣ�
��2���������Ʊ��ʵ�ԭ������������������еĶ�����̼��Ӧ����Ӧ�ķ���ʽ�ǣ�CO2+2NaOH=Na2CO3+H2O������̼������������������Һ��������������Һ����Ӧ�������������ƺ�̼��ƣ���̼�ᱵ�����ȳ�ȥ������̼���ƣ���û�������µ����ʣ�����Ҫ��ȥ��Һ�б������ɵ����ʣ�ʵ�鷽���ǣ�����������ʯ��ˮ������������Һ�����ˣ�
�ʴ�Ϊ����1����B����ǡ���кͣ�Na+��H+��ϡ������ܶȣ���2��CO2+2NaOH=Na2CO3+H2O��CD��
���� �����ǿ����кͷ�Ӧ��������ҺpH�ı仯����ģ�����Ҫ֪�������мӼ������м���ʱ����ҺpH�ı仯������ȷ��ָͬʾ���ı�ɫ��Χ��

| A�� | �����е����������� | B�� | ����������¯������pH�� | ||
| C�� | ���Ͻ��Ŵ��ȸ����Ŵ�����ʴ | D�� | ����KNO3��NH4NO3Ӫ��Ԫ������� |
| ��� | ����������� | �����Լ� |
| A | ����ˮ�����������Һ | �������� |
| B | �������ʳ�ι��� | ˮ |
| C | ϡ�������Ȼ�����Һ | ��̪��Һ |
| D | һ����̼�������̼���� | �����ʯ��ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| �� �� | �� �� | �� �� �� �� | �� �� �� Ӧ |
| 101�桫102�� | 150�桫160�� ���� | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
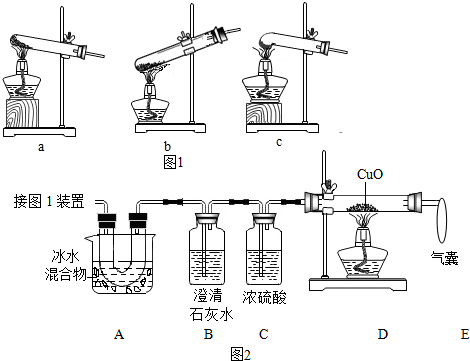
��2��ͼ2����֤�ȷֽ�����к� CO��CO2��װ�ã�
��װ��A����Ҫ�����dz�ȥ������������ֹ�Զ�����̼�ļ���������ţ�
�����ҵ��������ռ�δ��Ӧ��һ����̼����ֹ������Ⱦ��
��֤������CO2��������Bװ���ڵij���ʯ��ˮ����ǣ�B�з�Ӧ�Ļ�ѧ����ʽCO2+Ca��OH��2�TCaCO3��+H2O��
��֤������CO��������Dװ���к�ɫ�����죮
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��һ����̼û��ȫ��������ͭ��Ӧ����дһ�����ɣ�
��4����ȡ17.5g���ᾧ����Ʒ����50.00g��Һ����������ϡ���ᣬȻ��μ�KMnO4��Һ����KMnO47.9�ˣ�ǡ�÷�Ӧ��ȫ������֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
�������Ʒ�в��ᾧ�壨H2C2O4•2H2O ����������������д��������̣�
[�й����ʵ���Է���������Mr��H2C2O4��=90��Mr��H2C2O4•2H2O��=126��Mr��KMnO4��=158]��
| A�� | �������ۣ�ˮ������ԭ�Ӻ���ԭ�ӹ��ɵ� | |
| B�� | ����ת���ۣ�п������п�����ת�� | |
| C�� | ���ݽṹ�ۣ��ԭ�Ӻ�������������������ͬ����ѧ������ͬ | |
| D�� | �����غ�ۣ�10mL��������40%�����ᣬ��10mLˮ������������Ϊ20% |
��1�����룺ά����C���ܾ�������
��2��ʵ�鷽����
�ṩ�Լ���
ά����CƬ������ˮ����ɫʯ����Һ����ɫ��̪��Һ��ϡ���ᡢ����������Һ���Ȼ�����Һ��pH��ֽ��
���������Լ��������������������ַ�������д��ʵ������
| ʵ�����顡���ڡ����� | ʵ�����顡���֡����� |
| ������ά����CƬ��������ˮ�����Һ�������еμ���ɫʯ����Һ | ��Һ���ɫ |
| ������ά����CƬ��������ˮ�����Һ���ò�����պȡ����Һ����pH��ֽ�ϣ��Ժ���� | pHС��7 |
A������ͭ��B��ʳ�Ρ���C���������ơ�D������ͭ��
��4��ijͬѧ���뵽���������߲ˡ�ˮ���к��зḻ��ά����C������ʱ�䳤���Ƿ��ά����C�ĺ�������Ӱ�죮�����������ʵ�鷽����
�������������ͷ���һ�ܵ��������ֱ��飬��ɴ����֭Һ���������ձ��У�
��ȡ��֧ʢ��2mL��ɫ��ĵ�����Һ���Թܣ��ֱ�μ���������֭Һ���ӱ���ֱ����ɫ�պ���ʧ����¼�������£�
| ֭Һ | ������������֭Һ | ����һ�ܵ���������֭Һ |
| ���� | 12 | 20 |
������Ϊ��������ά����C�����ߵ������ʵ���������
������еõ�����ʾ�����������߲ˡ�ˮ���к��е�ά����C�����ŷ���ʱ���������������٣���ˣ�����Ҫ���������ʵ��߲ˡ�ˮ����
 ��1���û�ѧ������գ�
��1���û�ѧ������գ�