��Ŀ����
��ѧʵ���ǽ��п�ѧ̽������Ҫ��ʽ��
(1)����ʵ�������в������ڼ��ȵ���____������ĸ���ţ���
a���ձ� b����Ͳ c���Թ� d��������
(2)ij��ѧС��ѡ������װ�ú�ҩƷ����̽��ʵ�顣
 ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________________��
��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________________��
������A��B���ӣ�A�в��������岢����ʹB�е���Һ����ǣ�����Ϊʲô��
����Ҫ��ȡһƿ��Ϊ�����������CO2����ѡ��װ�õĵ��ܽӿڴ����ҵ���ȷ����
˳��Ϊ��a��________��_________��________��________��________��
(3)Ϊ̽����ҵ��ˮ���ۺ����ã�ij��ѧС����ʵ���������������ʵ�顣
 ��XΪ____���������ᱵ��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��XΪ____���������ᱵ��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
�ڲ���I�Ͳ������ж�Ҫ�õ�һ�ֲ����������������ڲ������е�������ʲô��
(4)ij�������Ϊ����̽���÷����������ȡ�����Ʒ�����з�����4.9 t��H2SO4��
��������Ϊ20%��������������м��Ӧ����ȡ����������ͬʱ�����ɵ�ȫ������ͨ������
����ͭ�в����ȣ�H2+CuO == Cu +H2O�����������������������ͭ��������
���𰸡�(1) b ��1�֣�
(2)��CaCO3 +2HCl == CaCl2 + CO2�� + H2O (1��)
��Ũ�����ӷ���ʹCO2�л���HC1����������CaCO3������ ��1�֣�
|

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���������װ�ö�������ʵ������ȡ������̼

��1��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ:____________________��
(2)����A��������__________________
��3)����ͼ6װȡ��ȡ������̼ʱ������©�����¶˹ܿڱ����û����Һ�У�������_____________��
(4)ͼ5��ͼ7װ�����.����ͼ5װ������ȡ������̼,ʱ��Ҫ�IJ�����_______(дһ�����ɣ�
��5)��ѧ��ȤС��Ϊ�˲ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ������ʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ��(��Ʒ�г�̼����⣬����ɷֲ������ᷴӦ��Ҳ���� ��ˮ).��ַ�Ӧ�����ˡ�ǧ�ٵȲ��������������������±�
| ϡ��������� | ʣ���������� |
| ��һ�μ���5g | 1.5g |
| �ڶ��μ���59 | 1.0g |
| �������59 | 0.5g |
| ���Ĵμ���59 | 0.3g |
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ_________________
��ԭϡ���������ʵ���������Ϊ����?(д��������̣�

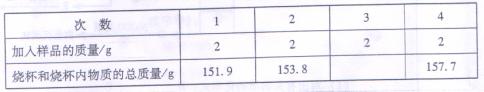

 �к���______���������ݵĻ�ѧ����ʽ��____________________________________��
�к���______���������ݵĻ�ѧ����ʽ��____________________________________��
 �������⣨��Ӧ�������ԣ���
�������⣨��Ӧ�������ԣ���