 25、 解: 3H2(g) + N2(g)
25、 解: 3H2(g) + N2(g)
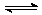 2NH3(g)
2NH3(g)
起始: 3mol 1mol 0mol
转化: 0.4mol 1.2mol 0.8mol
(1) NH3的平衡浓度 = 0.8/2mol/L
|
|
(2)
|
|
|
|
(3) H2的体积分数= 1.8/1.8+2.6+0.8 =56.25%
(4) P后/P前 = n后:n前 = 3.2:4 =4:5

 25、 解: 3H2(g) + N2(g)
25、 解: 3H2(g) + N2(g)
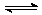 2NH3(g)
2NH3(g)
起始: 3mol 1mol 0mol
转化: 0.4mol 1.2mol 0.8mol
(1) NH3的平衡浓度 = 0.8/2mol/L
|
|
(2)
|
|
|
|
(3) H2的体积分数= 1.8/1.8+2.6+0.8 =56.25%
(4) P后/P前 = n后:n前 = 3.2:4 =4:5
