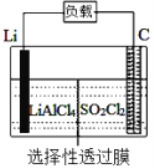
��Ŀ����
����Ŀ��������Ԫ��W��X��Y��Z��ԭ��������������m��P��q��r��s������ЩԪ����ɵĶ�Ԫ��������³�ѹ��rΪҺ�壬�����Ϊ��ɫ���塣m��Ħ������Ϊp��2����n��Ԫ��Y�ĵ��ʣ�����ɫֲ�������ò�������ɫ���壬p������ʹʪ��ĺ�ɫʯ����ֽ������q��ʹƷ����Һ��ɫ���������ʵ�ת����ϵ����ͼ��ʾ������˵����ȷ����

A. q��s��Ϊ���������� B. ԭ�Ӱ뾶��W��Y��X
C. Z���⻯���Y���⻯���ȶ� D. Z�ĺ�������һ��ǿ��
���𰸡�B
��������n��Ԫ��Y�ĵ��ʣ�����ɫֲ�������ò�������ɫ���壬����nΪ������YΪ��Ԫ�ء�p������ʹʪ��ĺ�ɫʯ����ֽ������pΪ������q��ʹƷ����Һ��ɫ��qΪ��������n����������p����������ӦӦ�õõ�NO��ˮ����ΪrΪҺ�壬����rΪˮ��sΪNO��m�ķ�������p����������2��������m�ķ�����Ϊ34�����ǵ�m��������Ӧ�õ�������������mһ��ΪH2S�����ϵõ�������Ԫ��W��X��Y��Z�ֱ�ΪH��N��O��S��s��NO���Dz�������������ѡ��A����ԭ�Ӱ뾶Ϊ��W��H����Y��O����X��N����ѡ��B��ȷ���⻯����ȶ��ԣ�H2O(Y���⻯��)��H2S(Z���⻯��)��ѡ��C����Z��S���ĺ����������֣�����������ǿ�ᣬ����������ǿ�ᣬѡ��D����

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�����Ŀ����������(POCl3)�������л��ϳɵ��Ȼ����ʹ�����
��֪����KSCN+AgNO3=AgSCN��+KNO3��
��Ksp(AgCl) > Ksp(AgSCN)��
�����������������
��PCl3��POCl3�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -111.8 | 74.2 | 137.5 | ��Ϊ��ɫҺ�壬��ˮ������ˮ�⣬���ɺ�������Ȼ��⣬�����ܡ� |
POCl3 | 2.0 | 105.3 | 153.5 |
ʵ������ȡPOCl3���ⶨ��Ʒ���ȵ�ʵ��������£�
��.�Ʊ�POCl3������������Һ̬PCl3�ķ�����ʵ��װ�ã����ȼ��г�װ��ʡ�ԣ����£�
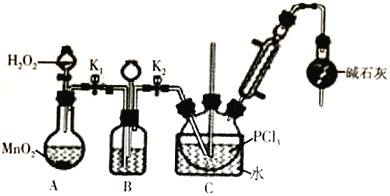
��1��װ��C��ʢװPCl3����������Ϊ_______________________����������PCl3�Ļ�ѧ����ʽΪ_______________________________��
��2��������װ��ɺ��װ��B�����Եķ�����_______________________________��
��3��װ��B��������________________________________���ش����㣩��
��4��װ��C�ķ�Ӧ�¶ȿ�����60-65��֮�䣬ԭ����____________________________________��
��.�ⶨ��Ʒ��POCl3���ȵ�ʵ�鲽�裺
��ʵ����������Ӧ����Һ����ȴ�����£�ȷ��ȡ1.3300 g��POCl3�ֲ�Ʒ�����ʲ�����Ԫ�أ�������ʢ��50.00mL����ˮ���ձ���ҡ������ȫˮ�⣬��ˮ��Һ���100.00mL��Һ��
��ȡ10.00mL��Һ����ƿ�У�����30.00mL0.1200mol��L-1AgNO3����Һ��
�ۼ�������������������ҡ�������ã�
�ܼ���ָʾ������0.1000 mol��L-1KSCN��Һ�ζ�������AgNO3��Һ�������յ�ʱ����ȥ12.00mLKSCN��Һ��
��5���ζ�������ѡ���ָʾ��Ϊ___________��Һ��
��6������۵�Ŀ����___________________________________________________��
��7����ò�Ʒ��POCl3�Ĵ���Ϊ__________%��
����Ŀ��������(Raney-Ni)��һ�ֶ�ṹ�������Ͻ𣬶���������ǿ�����ԣ���ϩ����Ȳ���⻯��Ӧ�ĸ�Ч������һ����ͭ����(��Ҫ��Cu��Fe��Co��Ni)��������������������:
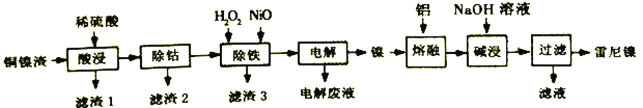
�±��г����йؽ������������������������pH:
�������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 7.7 |
������ȫ��pH | 3.3 | 9.9 | 9.2 |
��1���������ʱ����ͨ����������Ͻ��裬�ɽ�������1�������ܽ⣬���ӷ���ʽΪ___________��
��2����������ʱ���ȼ�������H2O2����Fe2+�����������ĵ�n(H2O2):n(Fe2+)=____���ټ���NiO�Ե�����Һ��pH��Ӧ����pH�ķ�ΧΪ__________________��
��3���������(�Զ��Բ������缫)��Ϊ�˻�õ��������������п�ѭ�����õ�������_______��
��4�����������Ϊ���γɶ�ṹ������������Ӧ�����ӷ���ʽΪ_________________��ʹ�����������������⻯��Ӧʱ����������Ҳ��ʵ���⻯��Ŀ�ģ�ԭ����____________________��
��5������Һ����Ҫ�ɷ������CO2��Ӧ�����ӷ���ʽΪ__________________________��