��Ŀ����
20��ˮ����Ҫ����Ȼ��Դ��������ķ�չ������أ���1��25��ʱ��ˮ�ܰ����з�ʽ���룺
H2O+H2O?H3O++OH- K1=1.0��10-14
OH-+H2O?H3O++O2- K2=1.0��10-36
ˮ��c��O2-��=1.0��10-36 mol•L-1mol•L-1������ֵ����
��2��ˮ�㷺Ӧ���ڻ�ѧ��Ӧ��������ĵ�������ۻ��δ����Ӧ���μ�һ��ˮ��������ɫ�ĵ����������õ���ɫ���壮�йظ�ʵ��Ľ��ͺ�������C��D��
A����ˮʹ��������γ���Һ
B��ˮ��������
C����������۷�Ӧ�Ƿ��ȷ�Ӧ
D��ˮ������
��3������ͭ��CuFe2O4���Ǻ���ǰ�����Ȼ�ѧѭ���ֽ�ˮ����IJ��ϣ�
����ij����С���Ʊ�����ͭ��CuFe2O4�����������£�

�����������Һ��Fe��NO3��3��Cu��NO3��2�����ʵ���Ũ�ȷֱ�Ϊ2.6mol•L-1��1.3mol•L-1��
�ٽ����������Һ��FeԪ�صĴ�����ʽ��Fe3+��Fe��OH��3���ѧʽ����
�ڽ����Ҫ���ٲ������̵�pH�仯���ڵμ�KOH��Һ��pH=4�Ĺ����У�������Һ������䣩��С��ͬѧ������Һ��c��Fe3+����c��Cu2+����pH�仯��������ͼ��������ȷ����B���á�A������B����գ�������֪��Ksp[Fe��OH��3]=2.6��10-39��Ksp[Cu��OH��2]=2.2��10-20��

�۲�����Ϊϴ�ӡ����
�������Ȼ�ѧѭ���ֽ�ˮ����Ĺ����У�����ͭ��CuFe2O4����Ҫ���ճ���ȱλ�壨CuFe2O4-a������ȱλֵ��a��Խ����Խ�ߣ�����Խ���ף�
����ȱλ����ˮ��Ӧ����Ļ�ѧ����ʽΪCuFe2O4-a+aH2O=CuFe2O4+aH2����
�ݿ���С�齫����ͭ��Ʒ��N2�������г�����գ�����ȱλ�������Ϊԭ������96.6%������ȱλֵ��a��=0.51��
���� ��1��ˮ��c��H3O+��=c��OH-��������K2=$\frac{c��{H}_{3}O��c��{O}^{2-}��}{c��O{H}^{-}��}$�����������ӵ����ʵ���Ũ�ȣ��ٸ���ˮ��������������Ӹ���֮��Ĺ�ϵ�б���ʽ���Ӷ��ó���ȷ���ۣ�
��2�������ĸ����Ƿ�Ӧǰ���������������ѧ���ʾ����䣬�ݴ����ش�
��3������������ˮ��Һ�з�����ˮ����������������
�������ܶȻ�������������������������ͭ������ȫ����ҺPH�����жϣ�
���������ǹ��˵õ�����������ϴ�Ӹ���������գ�
����ȱλ����ˮ��Ӧ�����������ͭ��CuFe2O4��������ԭ���غ���д��ƽ��
�ݽ�����ͭ��Ʒ��N2�������г�����գ�����ȱλ�������Ϊԭ������96.6%��CuFe2O4-a������ΪCuFe2O4��96.6%���ݴ���ʽ���㣻
��� �⣺��1��ˮ��c��H3O+��=c��OH-����K2=$\frac{c��{H}_{3}O��c��{O}^{2-}��}{c��O{H}^{-}��}$��c��O2-��=$\frac{{k}_{2}•c��O{H}^{-}��}{c��{H}_{3}{O}^{+}��}$=K2=1.0��10-36mol•L-1��
�ʴ�Ϊ��1.0��10-36 mol•L-1��
��2��������Ŀ��Ϣ��֪���������ۺ͵ⷴӦ��������ֻ�е⻯����˵��ˮû�вμӷ�Ӧ������û�м���ˮ֮ǰ�����ۺ͵�û��ʲô�仯������ˮ֮��Ӧ�ų�������ʹ����������ɫ��������˵����Ӧ�Ƿ��ȷ�Ӧ����Ӧ��������ֻ�е⻯����ɫ���壬˵��ˮ�ı��˸÷�Ӧ�����ʣ��ۺϷ�����ˮ��һ�ִ�����
��ѡC��D��
��3���ٽ����������Һ��Fe��NO3��3��Cu��NO3��2��������ˮ����������������������Ԫ�صĴ�����ʽΪFe3+��Fe��OH��3��
�ʴ�Ϊ��Fe��OH��3��
�ڽ����������Һ��Fe��NO3��3��Cu��NO3��2�����ʵ���Ũ�ȷֱ�Ϊ2.6mol•L-1��1.3mol•L-1��������ȫ������Ũ��С��10-5mol/L
��֪��Ksp[Fe��OH��3]=2.6��10-39��c��OH-��=$\root{3}{\frac{2.6��1{0}^{-39}}{1{0}^{-5}}}$=6.4��10-12mol/L��c��H+��=1.6��10-3mol/L��PH=3-lg1.6��2.8��ͼ���������ӳ�����ȫ��PH����Ksp[Cu��OH��2]=2.2��10-20����Һ������������Ũ��c��OH-��=$\sqrt{\frac{2.2��1{0}^{-20}}{1{0}^{-5}}}$=4.7��10-8mol/L��c��H+��=$\frac{1{0}^{-14}}{4.7��1{0}^{-8}}$=2.1��10-7mol/L��PH��6.7��ͼ��B���ϣ�
�ʴ�Ϊ��B��
���������ǹ��˵õ�����������ϴ�Ӹ���������գ�������Ϊϴ�ӡ����
�ʴ�Ϊ��ϴ�ӡ����
����ȱλ����ˮ��Ӧ����Ļ�ѧ����ʽΪCuFe2O4-a+aH2O=CuFe2O4+aH2����
�ʴ�Ϊ��CuFe2O4-a+aH2O=CuFe2O4+aH2����
�ݽ�����ͭ��Ʒ��N2�������г�����գ�����ȱλ�������Ϊԭ������96.6%��CuFe2O4-a������ΪCuFe2O4��96.6%��$\frac{CuF{e}_{2}{O}_{4-a}}{CuF{e}_{2}{O}_{4}}$��100%=96.6%
a=0.51��
�ʴ�Ϊ��0.51��
���� ���⿼�������ʷ����ᴿ�ķ����������ܶȻ������ļ�������жϣ�������ʵ������Ӧ�ã�ע�ⷴӦ������ԭ���غ������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

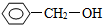 ��ͬ����ǣ�������
��ͬ����ǣ�������| A�� |  | B�� | 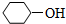 | C�� | CH3CH2OH | D�� | HOCH3 |
| A�� | Cs | B�� | Mg | C�� | Al | D�� | Cu |
| A�� | Dsԭ�������ڱ���λ�ڵ�7���ڵڢ�B�� | |
| B�� | Ds�ǹ���Ԫ�� | |
| C�� | Dsԭ�ӵĺ��������Ϊ110 | |
| D�� | DsΪ����Ԫ�� |
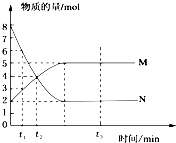 ��һ���¶��£�����������M��Nͨ���ݻ�ΪVL���ܱ������н��з�Ӧ��M��N�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��һ���¶��£�����������M��Nͨ���ݻ�ΪVL���ܱ������н��з�Ӧ��M��N�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | 0��t2����M��ʾ��ƽ����Ӧ������ $\frac{2}{{t}_{2}}$��mol•L-1•min-1�� | |
| B�� | t1��t2�������ڵ�ѹǿ��С | |
| C�� | �÷�Ӧ�ķ���ʽΪN?2M | |
| D�� | t2��t3ʱ�̵Ļ�������ƽ����Է���������� |
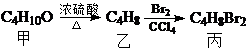 �����������ǣ�������
�����������ǣ�������| A�� | CH3CH2CHBrCH2Br | B�� | ��CH3��2CBrCH2Br | C�� | CH3CHBrCHBrCH3 | D�� | CH3CH ��CH2Br��2 |
| A�� | ����ȫȼ�գ�1 mol��ͪ�� ������ͪ�� ������ͪ�� ��������3 mol O2 ��������3 mol O2 | |
| B�� | �����顢������������黥Ϊͬ���칹�壬�е��������� | |
| C�� | ��ȩ�����ᡢ�����ƣ����ܷ���������Ӧ | |
| D�� | �Ҵ�����ͨ����ȥ��ȡ�����ӳɷ�Ӧ�������Ҷ��� |
| A�� | ���ǵ�ԭ�Ӻ�����Ӳ�����˵���������Ӷ����� | |
| B�� | ���ǵ��۷е���˵���������Ӷ����� | |
| C�� | ���ǵ��⻯����ȶ�����˵���������Ӷ���ǿ | |
| D�� | ���ʵ���ɫ��˵���������Ӷ����� |
 ��FԪ��ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p5��
��FԪ��ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p5��