��Ŀ����
5�� ��һ���¶��£�����������M��Nͨ���ݻ�ΪVL���ܱ������н��з�Ӧ��M��N�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��һ���¶��£�����������M��Nͨ���ݻ�ΪVL���ܱ������н��з�Ӧ��M��N�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | 0��t2����M��ʾ��ƽ����Ӧ������ $\frac{2}{{t}_{2}}$��mol•L-1•min-1�� | |
| B�� | t1��t2�������ڵ�ѹǿ��С | |
| C�� | �÷�Ӧ�ķ���ʽΪN?2M | |
| D�� | t2��t3ʱ�̵Ļ�������ƽ����Է���������� |
���� ͼ����Ӧ�ӿ�ʼ��ƽ�⣬N�����ʵ�����С��ӦΪ��Ӧ����ʵ����仯ֵΪ8mol-2mol=6mol��M�����ʵ������࣬ӦΪ����������ʵ����ı仯ֵΪ5mol-2mol=3mol������n��N����n��M��=6mol��3mol=2��1����֪��Ӧ�Ļ�ѧ����ʽΪ2N?M����Ϸ�Ӧ�ķ���ʽ�ɼ���������ʵķ�Ӧ�����Լ����ʵ���Ũ�ȹ�ϵ��
��� �⣺��ͼ���֪��Ӧ�Ļ�ѧ����ʽΪ2N?M��
A��0��t2����M��ʾ��ƽ����Ӧ������$\frac{\frac{5mol-2mol}{VL}}{{t}_{2}min}$=$\frac{3}{V{t}_{2}}$mol/��L•min������A����
B��t1��t2������������Ӧ2N?M��Nת��ΪM�����ʵ������٣����������ڵ�ѹǿ��С����B��ȷ��
C��N�����ʵ�����С��ӦΪ��Ӧ����ʵ����仯ֵΪ8mol-2mol=6mol��M�����ʵ������࣬ӦΪ����������ʵ����ı仯ֵΪ5mol-2mol=3mol������n��N����n��M��=6mol��3mol=2��1����֪��Ӧ�Ļ�ѧ����ʽΪ2N?M����C����
D��t2��t3ʱ�̵Ļ������������ʵ�����ͬ���ֱ�Ϊ8mol��7mol����ƽ����Է����������ȣ���D����
��ѡB��
���� ���⿼�黯ѧ��Ӧ���ʵ�Ӱ�������Լ�ƽ��ͼ����Ŀ����Ŀ�ѶȲ���ע�����ͼ�������ߵı仯�ص㣮

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�| A�� | ��A��Ԫ�� | B�� | +7��Ԫ�� | C�� | ������Ԫ�� | D�� | ����Ԫ�� |
| A�� | Ԫ��ԭ�ӵ������������������������� | |
| B�� | �����������ϼۡ����и����ϼ� | |
| C�� | Ԫ�ص������������һ������ԭ������������ | |
| D�� | ��������ľ���ֵ����ԭ���γ��ȶ��ṹ��������� |
 ��һ���¶��£�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯����������ͼ��ʾ�����������У���ȷ���ǣ�������
��һ���¶��£�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯����������ͼ��ʾ�����������У���ȷ���ǣ�������| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪ2 M?N | |
| B�� | t1ʱN��Ũ����MŨ�ȵ�2�� | |
| C�� | t2ʱ���淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬ | |
| D�� | t3ʱ����Ӧ���ʴ����淴Ӧ���� |
| A�� | CH3CO- | B�� | 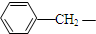 | C�� |  | D�� | R-CO- |
| A�� | Na | B�� | Mg | C�� | Ag | D�� | Fe |