��Ŀ����
10�� ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH $��_{H_{2}SO_{4}����}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/0C | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90-95�棬��E���ռ�90�����µ���֣�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75-77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ�Ũ��������ˮ�ų������ȣ��������Ž����ˣ�
��2�������ʯ�������Ƿ�ֹ����
��3������װ��ͼ�У�B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
a����ʪ b������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���²㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90-95�棬��ԭ���ǣ���֤����ȩ��ʱ��������ʹ��Ӧ������У��ֿɾ��������䱻��һ������
��7����ʵ���У�����ȩ�IJ���Ϊ51%
��8����֪����ȩ�ڼ��������¿��Ա�����������ͭ������д���÷�Ӧ�Ļ�ѧ����ʽ��CH3CH2CH2CHO+NaOH+2Cu��OH��2$\stackrel{��}{��}$CH3CH2CH2COONa+Cu2O��+3H2O��
���� ��1�����ܽ�Na2Cr2O7��Һ�ӵ�Ũ�����У���ΪŨ������ܶȴ��������Ž���
��2�������ʯ�������Ƿ�ֹ���У������Ⱥ���δ�ӷ�ʯ��Ӧ����ȴ�ӣ�
��3��B�����������Ƿ�Һ©����D����������ֱ�������ܣ�
��4����Һ©��ʹ��ǰ������еĵ�һ������Ǽ�©��
��5���ɱ������ݿ�֪������ȩ�ܶ�С��ˮ���ܶȣ��ݴ��жϣ�
��6��������Ŀ������Ӧ��Ͳ���ķе����ݿ�֪����Ӧ�¶ȱ�����90��95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��7��������ȩ�����۲���Ϊxg�����ݹ�ϵʽC4H10O��C4H8O�м�������۲��������ݲ���=$\frac{ʵ�ʲ���}{���۲���}$��100%���㣮
��8������ȩ�ڼ��������¿��Ա�����������ͭ�������ɶ����ƣ�������ͭ������ˮ��
��� �⣺��1����ΪŨ������ܶȴ��ܽ�Na2Cr2O7��Һ�ӵ�Ũ�����У��������Ž����ˣ�
�ʴ�Ϊ�����ܣ�Ũ��������ˮ�ų������ȣ��������Ž����ˣ�
��2�������ʯ�������Ƿ�ֹ���У�
�ʴ�Ϊ����ֹ���У�
��3��B�����������Ƿ�Һ©����D����������ֱ�������ܣ�
�ʴ�Ϊ����Һ©����ֱ�������ܣ�
��4����Һ©��ʹ��ǰ������еĵ�һ������Ǽ�©��
�ʴ�Ϊ��c��
��5������ȩ�ܶ�Ϊ0.8017 g•cm-3��С��ˮ���ܶȣ��ʷֲ�ˮ�����·���
�ʴ�Ϊ���£�
��6��������Ŀ������Ӧ��Ͳ���ķе����ݿ�֪����Ӧ�¶ȱ�����90��95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
�ʴ�Ϊ����֤����ȩ��ʱ��������ʹ��Ӧ������У��ֿɾ��������䱻��һ��������
��7��������ȩ�IJ���Ϊx������������������Ϊx�����ݹ�ϵʽ��
C4H10O��C4H8O
74 72
4xg 2g
��ã�x=$\frac{74��2}{72��4}$��100%=51%��
�ʴ�Ϊ��51��
��8������ȩ�ڼ��������¿��Ա�����������ͭ�������ɶ����ƣ�������ͭ������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2CH2CHO+NaOH+2Cu��OH��2$\stackrel{��}{��}$CH3CH2CH2COONa+Cu2O��+3H2O��
�ʴ�Ϊ��CH3CH2CH2CHO+NaOH+2Cu��OH��2$\stackrel{��}{��}$CH3CH2CH2COONa+Cu2O��+3H2O��
���� ���⿼���л���ѧʵ�顢��Ӧԭ����������������ѧ����ȣ��ѶȲ���ע���������������ת���ʵ�������ȩ�IJ��ʣ�ע��Ի���֪ʶ���������գ�

| A�� | ԭ��ص������͵��ص������������ķ�Ӧ�ֱ���������Ӧ����ԭ��Ӧ | |
| B�� | �¶Ȳ��䣬�ö��Ե缫��ⱥ��̼������Һ��ͨ��һ��ʱ�䣬��Һ��Ũ�Ȳ��䣬�о������� | |
| C�� | �ö��Ե缫���CuCl2��Һ��һ��ʱ���Ҫ�ָ�ԭ����Ũ�ȣ�Ӧ�������CuSO4 | |
| D�� | �Զ��Ե缫���CuSO4��Һ���������ϲ�������������ʵ���Ϊ0.010mol��������������Cu������Ϊ12.8g |
�±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩��
| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3+ | 1.1 | 3.2 |
| Al3+ | 3.0 | 5.0 |
| Fe2+ | 5.8 | 8.8 |
a����FeSO4��Һ��Na2CO3��Һͬʱ���뵽��Ӧ������
b����FeSO4��Һ�������뵽ʢ��Na2CO3��Һ�ķ�Ӧ������
c����Na2CO3��Һ�������뵽ʢ��FeSO4��Һ�ķ�Ӧ������
��2�����ɵ�FeCO3�����辭���ϴ�ӣ�����ϴ���Ƿ���ȫ�ķ�����ȡ���һ�ε�ϴ����Һ1��2mL���Թ��У������еμ��������ữ��BaCl2��Һ�����ް�ɫ�����������������ϴ�Ӹɾ���
��3�����Ƶõ�FeCO3���뵽������������Һ�У��ټ����������ۣ�80���½��跴Ӧ��
�����۵������Ƿ�ֹ+2�۵���Ԫ�ر�������
�ڷ�Ӧ������������ˣ���ȥ�������۵ķ����Ǽ������������������۷�Ӧ��ȫ��
��4�������Һ��Ũ��������������ˮ�Ҵ������á����ˡ�ϴ�ӡ��������������������壮��������м�����ˮ�Ҵ���Ŀ���ǽ���������������ˮ�е��ܽ����������ھ���������
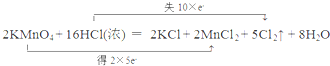
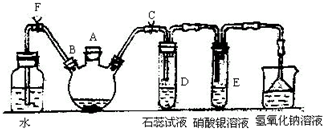 ʵ�����Ʊ��屽������ͼ��ʾ��װ�ã���д���пհף�
ʵ�����Ʊ��屽������ͼ��ʾ��װ�ã���д���пհף�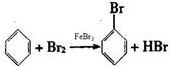 ��
��