��Ŀ����
5��ij����ʯ��Ʒ����Ҫ�ɷ�Ϊ�������������������SiO2��ij��ѧ��ȤС��Ը�����ʯ����Ԫ�صļ�̬��������������̽������1��̽������ʯ����Ԫ�صļ�̬
��������裺
����һ��������ʯ����Ԫ�صļ�̬Ϊ+2��
�������������ʯ����Ԫ�صļ�̬Ϊ+3��
��������������ʯ����Ԫ�صļ�̬Ϊ+2��+3��
�����ʵ�鷽����
Ϊ��ȷ��������ʯ����Ԫ�صļ�̬����ͬѧ���������ʵ�鷽��������������ѡ�Լ�������ɸ�̽�����̣���ѡ�Լ���1mol?L-1H2SO4��Һ��3%˫��ˮ��Һ��2mol?L-1HNO3��Һ��0.01mol?L-1���������Һ��1mol?L-1NaOH��Һ��0.1mol?L-1KI��Һ��0.01mol?L-1KSCN��Һ������ˮ��
| ʵ�鲽�� | Ԥ�������� |
| ����1��ȡ������Ʒ���Թ��У��μ�������1mol?L-1H2SO4��Һ�������ܽ⣬�õ�A��Һ | |
| ����2�� | �����������Һ�Ϻ�ɫ��ȥ������Ʒ�к�+2�۵���Ԫ�� |
| ����3�� | ����Һ���ɫ������Ʒ�к�+3�۵���Ԫ�� |
��ͬѧ���������ʵ�鷽����ȡ������Ʒ���Թ��У��μ�������2mol?L-1HNO3��Һ�������ܽ⣬�õ�B��Һ��ȡ����B��Һ���Թ��У������еμӼ���0.01mol?L-1KSCN��Һ����Һ���ɫ��˵��������ʯ����Ԫ�صļ�̬Ϊ+3������Ϊ�˽��۴������ȷ����������������ϡ�������ǿ�����ԣ��ܽ���Ʒ��+2�۵�������Ϊ+3�۵�����
��2��̽������ʯ�����ĺ���
��2.25g������ʯ��Ʒ��һϵ�л�ѧ�������Ƶ���Ԫ��ȫ��ΪFe2+�Ĵ���Һ250mL��������0.02mol?L-1���Ը��������Һ������ʯ����Ԫ�صĺ������вⶨ��
��ȡ25.00mL����Һ����ƿ�У��������Ƶ�0.02mol?L-1���Ը��������Һ���еζ����жϴﵽ�ζ��յ�ķ����ǵμ����һ�����Ը��������Һʱ����Һ��dz��ɫͻȻ��Ϊ��ɫ���Ұ�����ڲ��ٱ�Ϊ��ɫ�����ﵽ�ζ��յ㣮
���ظ��˵ζ�����2��3�Σ�ƽ���������Ը��������Һ21.50mL���������ʯ����Ԫ�ص���������Ϊ53.5%��
���� ��1����������������Ԫ�صļ�̬Ϊ+2��+3����+2��+3��
�ڼ����������ӣ��ø��������Һ�������������ɫ��˵�������������ӣ�������������KSCN��Һ�������ɫ˵�����������ӣ�
��ϡ�������ǿ�����ԣ��������������ܽ⣻
��2����KMnO4��Һ����ɫ������������ָʾ����
�ڸ��ݷ�Ӧ5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O���ɵó�5Fe2+��MnO4-������ MnO4-��Һ���Ϊ21.50mLʱ�������Ӧ���������ӵ����ʵ����������250mL��Һ���������ӵ����ʵ�������������������������
��� �⣺��1����������������Ԫ�صļ�̬Ϊ+2��+3����+2��+3����������������ʯ����Ԫ�صļ�̬Ϊ+2��+3���ʴ�Ϊ��������ʯ����Ԫ�صļ�̬Ϊ+2��+3��
�ڼ����������ӣ��ø��������Һ����ȡ2mLA��Һ���Թ��У��ý�ͷ�ιܵμ�1��2��0.01mol•L-1KMnO4��Һ�����Թܣ������������ɫ��˵�������������ӣ�������������KSCN��Һ��ȡ2mLA��Һ���Թ��У��ý�ͷ�ιܵμ�1��2��20%KSCN��Һ�����Թܣ�����Һ���ɫ˵�����������ӣ��ʴ�Ϊ��
| ʵ�鲽�� | Ԥ�������� |
| ����2��ȡ��������1�����õ�A��Һ���Թ��У��μӼ���0.01mol?L-1���������Һ���� | ���������Һ�Ϻ�ɫ��ȥ |
| ����3��ȡ��������1�����õ�A��Һ���Թ��У��μӼ���0.01mol?L-1KSCN��Һ���� | ��Һ���ɫ |
��2����KMnO4��Һ�ʺ�ɫ��Fe2+��Ӧ��ϣ��������һ��KMnO4��Һ����ɫ����ȥ��˵���ζ����յ㣬�ʴ�Ϊ���μ����һ�����Ը��������Һʱ����Һ��dz��ɫͻȻ��Ϊ��ɫ���Ұ�����ڲ��ٱ�Ϊ��ɫ�����ﵽ�ζ��յ㣻
�ڸ��ݷ�Ӧ5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O���ɵó�5Fe2+��MnO4-������ MnO4-��Һ���Ϊ21.50mLʱ����MnO4-���ʵ���Ϊ��0.02mol/L��0.0215L=0.00043mol������ʯ����Ԫ�صİٷֺ����ǣ�$\frac{0.00043mol��5��56g/mol��\frac{250}{25}}{2.25g}$��100%=53.5%���ʴ�Ϊ��53.5%��
���� ���⿼����ʵ����ƵĻ���ԭ�������ӵļ��鷽�������ʺ����IJⶨʵ�鷽����ƣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�������ͼ���������ע����ճ������ӵļ��鷽����

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�| A�� | ClO3-��Cl-��K+ | B�� | ClO-��Cl-��H+ | ||
| C�� | NaClO��NaClO3��NaNO3 | D�� | NaClO��Na2SO4��NaCl |
����ˮ ��������Һ ��ʯ��ˮ ����ˮ �������� ��NaOH��Һ ��FeSO4��Һ ��FeCl3��Һ ��CuSO4��Һ ����ᣮ
| A�� | �ڢۢܢ�� | B�� | �ݢߢ��� | C�� | �ڢۢܢݢ� | D�� | �٢ޢ� |
��1��amol ��2��bmol��3��$\frac{a}{3}$mol��4��$\frac{b}{3}$mol��5��0mol��6����4a-b��mol��
| A�� | ��1����2����4����5����6�� | B�� | ��1����3����4����5����6�� | C�� | ��1����2����3����5����6�� | D�� | ֻ�У�1����3����5�� |
 ���ڱ���ǰ������Ԫ��R��W��X��Y��Z ��ԭ���������ε�����R�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ1��W���⻯��ķе��ͬ������Ԫ���⻯��ķе�ߣ�X2+��W2-������ͬ�ĵ��Ӳ�ṹ��YԪ��ԭ�ӵ�3P�ܼ����ڰ����״̬��Z+�ĵ��Ӳ㶼�������ӣ���ش��������⣺
���ڱ���ǰ������Ԫ��R��W��X��Y��Z ��ԭ���������ε�����R�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ1��W���⻯��ķе��ͬ������Ԫ���⻯��ķе�ߣ�X2+��W2-������ͬ�ĵ��Ӳ�ṹ��YԪ��ԭ�ӵ�3P�ܼ����ڰ����״̬��Z+�ĵ��Ӳ㶼�������ӣ���ش��������⣺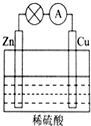 ��1����ͼ��ʾ��ԭ���װ���У��为��������Zn���������ܹ��۲쵽��������ͭƬ���������ɫ���ݣ������ĵ缫��Ӧʽ��2H++2e-=H2����ԭ��ع���һ��ʱ���������п6.5g����ų�����0.2g��
��1����ͼ��ʾ��ԭ���װ���У��为��������Zn���������ܹ��۲쵽��������ͭƬ���������ɫ���ݣ������ĵ缫��Ӧʽ��2H++2e-=H2����ԭ��ع���һ��ʱ���������п6.5g����ų�����0.2g��