��Ŀ����
5����1������ѧ��ѧ�Ľ��������У������Ũ����������лᷢ���ۻ��������Fe��Al���ѧʽ������2��д������Ũ���ᣨ�������ڼ��������·�Ӧ�Ļ�ѧ����ʽ2Fe+6H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe2��SO4��3+3SO2��+6H2O��
��3�����ֱ�������������Һ��ϡ���ᷴӦ��������ͬ����µõ���ͬ��������壬��������Ӧ������������������������ʵ���֮��Ϊ2��3��
���� ��1�������£�Fe��Al���䡢Ũ�����Ũ���ᷢ���ۻ����Դ������
��2��Ũ�������ǿ�������ԣ��ܺͽ������ڼ��������·�Ӧ��
��3����2Al+2NaOH+2H2O=2NaAlO2+3H2����2Al+3H2SO4=Al2��SO4��3+3H2���ɵ�2NaOH��3H2SO4��3H2��
��� �⣺��1��������Fe��Al�ܱ�Ũ�����Ũ����ۻ����������ܵ���������ֹ��Ӧ�Ľ�һ�����У��ʴ�Ϊ��Fe��Al��
��2��Ũ�������ǿ�������ԣ��ܺͽ������ڼ��������·�Ӧ������2Fe+6H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe2��SO4��3+3SO2��+6H2O���ʴ�Ϊ��2Fe+6H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe2��SO4��3+3SO2��+6H2O��
��3����2Al+2NaOH+2H2O=2NaAlO2+3H2����2Al+3H2SO4=Al2��SO4��3+3H2����֪�����ų�������������ʱ��n��NaOH����n��H2SO4��=2��3���ʴ�Ϊ��2��3��
���� ���⿼����������������ʼ����㣬���ݻ�ѧ����ʽ�ҳ�2NaOH����3H2SO4��3H2������ϵ�ǽ���Ĺؼ��������Ѷ����У�Ҫ��ѧ����ʽ��дȷ������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
16����֪��C��s��+O2��g��=CO2��g����H1
CO2��g��+C��s��=2CO��g����H2
2CO��g��+O2��g��=2CO2��g����H3
4Fe��s��+3O2��g��=2Fe2O3��s����H4
3CO��g��+Fe2O3��s��=3CO2��g��+2Fe��s����H5
���й���������Ӧ�ʱ���ж���ȷ���ǣ�������
CO2��g��+C��s��=2CO��g����H2
2CO��g��+O2��g��=2CO2��g����H3
4Fe��s��+3O2��g��=2Fe2O3��s����H4
3CO��g��+Fe2O3��s��=3CO2��g��+2Fe��s����H5
���й���������Ӧ�ʱ���ж���ȷ���ǣ�������
| A�� | ��H1��0����H3��0 | B�� | ��H2��0����H4��0 | C�� | ��H2=��H1+��H3 | D�� | ��H3=$\frac{V{H}_{4}+2V{H}_{5}}{3}$ |
13�������ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±�������
����ݱ�����Ϣ��д��
��1��XԪ�������ڱ��е�λ���ǵڶ����ڵ�IVA�壬����Է���������С����̬�⻯��Ļ�ѧʽ��CH4��
��2��Y���Ӱ뾶��Z���ӵİ뾶�����С������YԪ�ص�����������ˮ�����ZԪ�ص�������������Ӧ�����ӷ���ʽ�ǣ�Al2O3+2OH-=2AlO2-+H2O��
��3��X��M��Ԫ�ص���̬�⻯����ȶ��Ը�ǿ����HCl���ѧʽ�������پٳ�һ��ʵ���Ƚ�M��X��Ԫ�صķǽ�����ǿ�����û�ѧ����ʽ��ʾ��Na2CO3+2HClO4=2NaClO4+CO2��+H2O��
| Ԫ�� | �ṹ�����ʵ���Ϣ |
| X | �����л���ĺ���Ԫ�أ���Ԫ�ص�һ�����������̬�⻯�ﶼ�ǵ��͵��������� |
| Y | �������У���ϡ�������⣩ԭ�Ӱ뾶����Ԫ�أ��õ���Һ̬ʱ�������˷�Ӧ�ѵĴ��Ƚ��� |
| Z | ��Yͬ���ڣ�������������ˮ��������� |
| M | ��ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�����ɱ���� |
��1��XԪ�������ڱ��е�λ���ǵڶ����ڵ�IVA�壬����Է���������С����̬�⻯��Ļ�ѧʽ��CH4��
��2��Y���Ӱ뾶��Z���ӵİ뾶�����С������YԪ�ص�����������ˮ�����ZԪ�ص�������������Ӧ�����ӷ���ʽ�ǣ�Al2O3+2OH-=2AlO2-+H2O��
��3��X��M��Ԫ�ص���̬�⻯����ȶ��Ը�ǿ����HCl���ѧʽ�������پٳ�һ��ʵ���Ƚ�M��X��Ԫ�صķǽ�����ǿ�����û�ѧ����ʽ��ʾ��Na2CO3+2HClO4=2NaClO4+CO2��+H2O��
17�������л���������϶�������ǣ�������
| A�� | 3-��-2-��ϩ | B�� | 3-��-2-��ϩ | C�� | 2��2-�������� | D�� | 2-��-1-��Ȳ |
15����ѧ�㷺Ӧ������������������˵����ȷ���ǣ�������
| A�� | ��ͭ����Ӳ�Ҷ����ڴ����� | |
| B�� | �����Ǻ�������Һ����������Ӧ�������ƾ��� | |
| C�� | ��·�õ�������Ҫ����C��H��O��Ԫ�� | |
| D�� | �������м��뵨������ʹ�����ʷ������� |
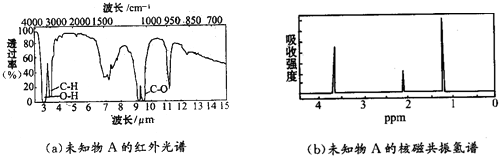
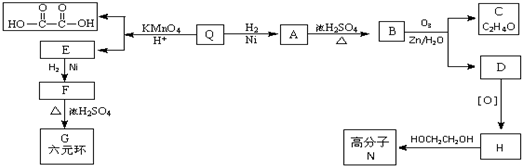
 ��Q�ķ�ʽ�ṹ��ʽ��
��Q�ķ�ʽ�ṹ��ʽ�� ��
�� ��
��