��Ŀ����
13����Ԫ�����ڱ��У�һϡ������Ԫ��ԭ�ӵ��������ӹ���Ϊ4s24p6������ͬ���ڵ�A��B��C��D����Ԫ�أ����ǵ�ԭ�ӵ���������������Ϊ2��2��1��7������A��C��Ԫ��ԭ�ӵĴ���������Ϊ8��B��D��Ԫ��ԭ�ӵĴ���������Ϊ18��E��D��Ԫ�ش���ͬ�壬���ڸ���Ԫ���У�E����̬�⻯��ķе���ߣ���1��BԪ�������ڱ��е�λ�õ������ڢ�B�壮
��2��E����̬���⻯����ͬ��Ԫ���зе���ߵ�ԭ����HF���Ӽ����������ƻ�����Ҫ�ϸߵ�������
��3��A��C��Ԫ�ص�һ������Ca��K������Ԫ�ط��ţ�
���� ��Ԫ�����ڱ��У�ijϡ������Ԫ��ԭ�ӵ��������ӹ���Ϊ4s24p6�����ڵ������ڣ�����ͬ���ڵ� A��B��C��D����Ԫ�أ����ǵ�ԭ����������������Ϊ2��2��1��7������A��C��Ԫ��ԭ�ӵĴ���������Ϊ8����AΪCa��CΪK��B��D��Ԫ��ԭ�ӵĴ���������Ϊ18����BΪZn��DΪBr��E��D��Ԫ�ش���ͬ�壬���ڸ���Ԫ���У�E����̬�⻯��ķе���ߣ���EΪF���ݴ˽��
��� �⣺��Ԫ�����ڱ��У�ijϡ������Ԫ��ԭ�ӵ��������ӹ���Ϊ4s24p6�����ڵ������ڣ�����ͬ���ڵ� A��B��C��D����Ԫ�أ����ǵ�ԭ����������������Ϊ2��2��1��7������A��C��Ԫ��ԭ�ӵĴ���������Ϊ8����AΪCa��CΪK��B��D��Ԫ��ԭ�ӵĴ���������Ϊ18����BΪZn��DΪBr��E��D��Ԫ�ش���ͬ�壬���ڸ���Ԫ���У�E����̬�⻯��ķе���ߣ���EΪF��
��1��BΪZnԪ�أ���������Ų�ʽΪ��1s22s22p63s23p63d104s2���������ڱ��е������ڢ�B�壬
�ʴ�Ϊ���������ڢ�B�壻
��2��HF���Ӽ����������ƻ�����Ҫ�ϸߵ����������Էе�ϸߣ�
�ʴ�Ϊ��HF���Ӽ����������ƻ�����Ҫ�ϸߵ�������
��3��CaԪ��ԭ��4s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϴ�һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Ca��K��
�ʴ�Ϊ��Ca��K��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų�������������ܵȣ��ؼ����պ�������Ų����ɣ�ע������ͬ����Ԫ�ص�һ�������쳣�����

| A�� | ������Һ�м�������İ�ˮ��Al3++4OH-�TAlO2-+2H2O | |
| B�� | ͭ����98.3%����������·�Ӧ Cu+4H++SO42-$\frac{\underline{\;\;��\;\;}}{\;}$Cu2++SO2��+2H2O | |
| C�� | �Ȼ�������Һ�м������3Fe2++4H++NO3-�T3Fe3++2H2O+NO�� | |
| D�� | ����ʯ��ˮ�м���������NaHCO3��Һ��Ca2++2OH-+2HCO3-�TCaCO3��+2H2O+CO32- |
| A�� | H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$H2O+Cu | B�� | CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2�� | ||
| C�� | Cu+4HNO3�TCu��NO3��2+2NO2��+2H2O | D�� | CaCO3+2HCl�TCaCl2+H2O+CO2�� |
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2 800 | 2 050 | 714 | 191 |
��2������ʱ�����Al2O3�������AlCl3��ԭ�����ǹ��ۻ��������̬ʱ�����룬�ѵ��磮
| A�� | ��������¥����һ�����ѧ�ͽ���������ص���Ȼ���� | |
| B�� | ���ƹܵ�Ϳ���ۿɷ���ʴ | |
| C�� | ﮿��������������ᡢ�ݵ�����Ŀɳ���� | |
| D�� | ������Ǧ������������۵� |
| A�� | ��HCl�ӳ�����CH3COCl | B�� | ��H2O�ӳ�����CH3COOH | ||
| C�� | ��CH3COOH�ӳ�����CH3COOCOCH3 | D�� | ��CH3OH�ӳ�����CH3COCH2OH |
 ��
�� �����ɼ��Լ��γɵķǼ��Է��ӵĽṹʽΪ
�����ɼ��Լ��γɵķǼ��Է��ӵĽṹʽΪ ���÷�������ԭ�ӵ��ӻ����������sp3�ӻ������⻯��ķе���ߵ���H2O��
���÷�������ԭ�ӵ��ӻ����������sp3�ӻ������⻯��ķе���ߵ���H2O��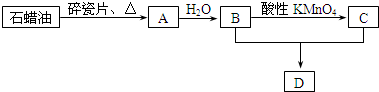
 CH3CH2OH��Ӧ�����Ǽӳɷ�Ӧ��B+C��D�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH
CH3CH2OH��Ӧ�����Ǽӳɷ�Ӧ��B+C��D�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH  CH3COOC2H5+H2O����Ӧ������������Ӧ��ȡ����Ӧ��
CH3COOC2H5+H2O����Ӧ������������Ӧ��ȡ����Ӧ��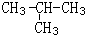 ��
��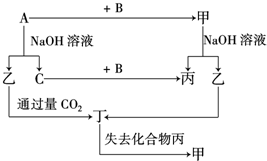 �ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪CΪ�ܶ���С�����壬���ǵ���ʣ�
�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪CΪ�ܶ���С�����壬���ǵ���ʣ�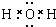 ��
��