��Ŀ����
����Ŀ�����仯���������ºϽ�ҵ���������졢����ȼ�ϵȷ����й㷺Ӧ�á�
��1�����̬ԭ�ӵĵ����Ų�ʽΪ_____��������ɷֱ��γ�[BF4]-��[AlF6]3-��[BF4]-�Ŀռ乹��Ϊ___����Ԫ�ز������γ�[BF6]3-��ԭ����________________��
��2��B2H6��һ�ָ���ȼ�ϣ�����Cl2��Ӧ���ɵ�BCl3�����ڰ뵼����ӹ��ռ��ߴ������졣�ɵڶ�����Ԫ����ɵ���BCl3��Ϊ�ȵ������������Ϊ________���������ӵ�����ԭ���ӻ���ʽΪ_________��
��3��������(H3N��BH3)��Ti(BH4)3��Ϊ���ܹ�ע�����ʹ�����ϡ�
��B��N�ĵ縺��:B______N(��������������������������ͬ)��
��Ti(BH4)3��TiCl3��LiBH4��Ӧ�Ƶá�д���Ʊ���Ӧ�Ļ�ѧ����ʽ:_________��
��4������(BP)���ܵ��߶ȹ�ע����ĥ���ϣ�����������������ı����㡣��ͼΪ������
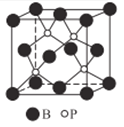
������������______����(�������)���Ƿ�����λ��? _____ (����������������)
�ھ�����Pԭ�ӵ���λ��Ϊ_____��
����֪BP�ľ����߳�Ϊanm��NAΪ�����ӵ���������ֵ������������ܶ�Ϊ_____g��cm-3(�ú�a��NA��ʽ�ӱ�ʾ)��
���𰸡� 1s22s22p1 �������� ��ԭ�ӵļ����Ӳ�ֻ��2s��2p�ĸ�ԭ�ӹ�����������γ��ĸ����ۼ� CO32-��NO3- sp2 �� TiCl3+3LiBH4==Ti(BH4)3+3LiCl ԭ�� �� 4 ![]()
��������������BΪ5��Ԫ�ء����Ը�������ԭ�Ӽ۲���Ӷ����ж���ռ乹�͡����ݵȵ���ԭ��Ѱ�Һ��ʵĵȵ����塣���Ը�������ԭ�ӵļ۲���Ӷ����ж����ӻ���ʽ��Ԫ�صķǽ�����Խǿ����縺��Խǿ�����ݾ����Ӳ�ȿ����ж�����ܵľ������͡�
��⣺��1�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p1��������ɷֱ��γ�[BF4]-��[AlF6]3-��[BF4]-�Ŀռ乹��Ϊ�������壬��Ԫ�ز������γ�[BF6]3-��ԭ������ԭ�ӵļ����Ӳ�ֻ��2s��2p�ĸ�ԭ�ӹ����û��d��������������γ��ĸ����ۼ���
��2���ɵڶ�����Ԫ����ɵ���BCl3��Ϊ�ȵ������������ΪCO32-��NO3-���������ӵ�����ԭ�ӵļ��ܲ���Ӷ���Ϊ4�������ӻ���ʽΪsp2��
��3����B�ķǽ����Ա�N�����ʵ縺��:B��N��
��Ti(BH4)3��TiCl3��LiBH4��Ӧ�Ƶã��÷�Ӧ�Ļ�ѧ����ʽΪTiCl3+3LiBH4==Ti(BH4)3+3LiCl��
��4��������(BP)����ĥ���ϣ���Ӳ�ȱ�Ȼ�ܴ�����������ԭ�Ӿ��塣�ɾ����ṹ��֪��B��P����λ����Ϊ4����Bԭ�������ֻ��3�����ӡ���Pԭ���йµ��Ӷԣ���Bԭ����Pԭ��֮������γ���λ�������бض�������λ����
�ھ�����Pԭ�ӵ���λ��Ϊ4��
��BP�ľ����߳�Ϊanm���������Ϊ![]() ���ɾ����ṹʾ��ͼ��֪��ÿ�������к���4��Pԭ�Ӻ�4��Bԭ�ӣ���NA������������Ϊ168g������������ܶ�Ϊ
���ɾ����ṹʾ��ͼ��֪��ÿ�������к���4��Pԭ�Ӻ�4��Bԭ�ӣ���NA������������Ϊ168g������������ܶ�Ϊ![]() g��cm-3��
g��cm-3��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�����Ŀ������Ǧ������Ǧ���ء���ά���ؼ���Ϳ�Ϸ����Լ�����ҵ��ͨ���÷�Ǧ��(��Ҫ�ɷ�ΪPbS)��������Ǧ�������������£�

��֪��
��25�棬Ksp(PbS)=1.0��10-28��Ksp(PbCl2)=1.6��10-5
��PbCl2(s)+2Cl-(aq)![]() PbCl42-(aq) ��H>0
PbCl42-(aq) ��H>0
��Fe3+������������ʽ��ʼ����ʱ��pHֵΪ1.9
(1)����Ksp(PbS)��Ksp(PbCl2)��PbS+2HCl![]() PbCl2+H2S�ķ�Ӧ�̶Ⱥ�С������FeCl3������Ӧ�̶ȣ�ԭ����____________________������ٷ�Ӧ�����пɹ۲쵽�е���ɫ�������ɣ��ܷ�Ӧ�����ӷ���ʽΪ_____________________���ò����������Һ��pH<1.9����ҪĿ����_______________________��
PbCl2+H2S�ķ�Ӧ�̶Ⱥ�С������FeCl3������Ӧ�̶ȣ�ԭ����____________________������ٷ�Ӧ�����пɹ۲쵽�е���ɫ�������ɣ��ܷ�Ӧ�����ӷ���ʽΪ_____________________���ò����������Һ��pH<1.9����ҪĿ����_______________________��
(2)������б���ʳ��ˮ��������_________________________��
(3)���������ҺA��������Ũ�����ñ�ˮԡ��ȴ�ᾧ������еIJ�����__________(���������)��
(4)������У�������ϡ�����ַ�Ӧ����������Һ��c(Cl-)=1.0mol��L-1����c(SO42-)=________[Ksp(PbSO4)=1.6��10-8]�����������ҺB�������Լ�X�������ѭ�����ã��Լ�XӦѡ�������е�_____(����)��
a.HNO3 b.Cl2 c.H2O2 d.����
(5)��Ǧ����Ǧ����ʹˮ�����ؽ���Ǧ�ĺ�����������������Ⱦ��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb(OH)+��Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��ǦŨ�ȷ���x����ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��

��̽��Pb2+�����ʣ���Pb2+����Һ����εμ�NaOH��Һ���ȱ���ǣ������μ�NaOH��Һ�ֱ���壬pHΪ13��14ʱ����Һ�з�������Ҫ��Ӧ�����ӷ���ʽΪ__________________��
�ڳ�ȥ��Һ�е�Pb2+������С����һ�������Լ�(DH)�������������ӣ��Ӷ�ȥ��ˮ�еĺ���Ǧ�������������ӣ�ʵ������¼���£�
���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ |
����ǰŨ��/(mg��L-l) | 0.100 | 29.8 | 0.12 | 0.087 |
������Ũ��/(mg�� L-1) | 0.004 | 22.6 | 0.04 | 0.053 |
�������Լ�(DH)����Ǧ��������Ҫ�����ķ�ӦΪ��2DH(s)+Pb2+(aq)![]() D2Pb(s)+2H+(aq)������Ǧʱ����ʵ�pHԼΪ_____________����ʵ����Ǧ���ѳ���Ϊ_________________��
D2Pb(s)+2H+(aq)������Ǧʱ����ʵ�pHԼΪ_____________����ʵ����Ǧ���ѳ���Ϊ_________________��