��Ŀ����
9�����г�����pH=2�������pH=2�Ĵ�����Һ�ң���������в����ش����⣺��1��������0.1mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ���������BD��
A��c��H+�� B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ C��c��H+��•c��OH-�� D��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$
��2��ȡ10mL������Һ������������ˮ������ĵ���ƽ�����ң�����������ҡ��������ƶ�����ȡ10mL������Һ������������ˮ�����ƹ��壨����������ǰ����Һ������ֲ��䣩���������ܽ����Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ �ı�ֵ����С�����������С������ȷ��������
��3��ȡ������ļס�������Һ���ֱ��õ�Ũ�ȵ�NaOHϡ��Һ�кͣ������ĵ�NaOH��Һ�������С��ϵΪ��V���ף��� V���ң��� ���������������=������
��4����֪25��ʱ��������ĵ���ƽ�ⳣ�����£�
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ��K1 | 1.8��10-5 | 4.3��10-7 | 3.0��10-8 |
| K2 | -- | 5.6��10-11 | -- |
A��HCO3- B��CO32- C��ClO- D��CH3COO-
��5�������£�ȡ����Һϡ��100������pH=4��ȡ99mL����Һ��1mL1mol/L��NaOH��Һ��ϣ�������Һ����仯�����ָ�������ʱ��pH=10��
���� ��1��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С��c��OH-������Kw���䣻
��2��������������ʣ���ˮϡ�ʹٽ�������룻������м�������ƹ��壬��Һ�д��������Ũ���������ƴ�����룻
��3��pH��ȵĴ�������ᣬ��������ʵ���Ũ�ȴ������
��4������ƽ�ⳣ��Խ����ĵ���̶�Խ����Һ����Խǿ�����ݱ������ݿ�֪��������ǿ������˳��Ϊ��CH3COOH��H2CO3��HClO��HCO3-��
��5��pH=2������ϡ��10n����pH����n��99mL0.01mol/L��������Һ��1mL1mol/L��NaOH��Һ�����Һ�Լ��ԣ�
��� �⣺��1��A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С����A����
B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$=$\frac{K}{c��C{H}_{3}CO{O}^{-}��}$��K���䣬���������Ũ�ȼ�С����ϡ�����б�ֵ���B��ȷ��
C��ϡ���̣��ٽ����룬c��H+����С��c��OH-������c��H+��•c��OH-��=Kw��Kw���䣬��C����
D��ϡ���̣��ٽ����룬c��H+����С��c��OH-���������ֵ���D��ȷ��
�ʴ�Ϊ��BD��
��2��������������ʣ���ˮϡ�ʹٽ�������룬���Դ������ƽ��������Ӧ�����ƶ���������м�������ƹ��壬��Һ�д��������Ũ���������ƴ�����룬��������Ũ�ȼ�С���������Ũ����������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$��С��
�ʴ�Ϊ�����ң���С��
��3��pH��ȵĴ�������ᣬ�����Ũ�ȴ������ᣬ�������pH�������ᣬ��������ʵ����������ᣬ������ʵ���Խ����Ҫ��Ũ�ȵ�����������Һ���Խ���������ĵ�NaOH��Һ�������С��ϵΪ��V���ף���V���ң���
�ʴ�Ϊ������
��4������ƽ�ⳣ��Խ����ĵ���̶�Խ����Һ����Խǿ�����ݱ������ݿ�֪��������ǿ������˳��Ϊ��CH3COOH��H2CO3��HClO��HCO3-������Խ������Ӧ��������ӽ�������ӵ�����Խǿ������������������ǿ��ΪCO32-���ʴ�Ϊ��B��
��5��pH=2������ϡ��10n����pH����n������ȡ����Һϡ��100����pHΪ4��99mL0.01mol/L��������Һ��1mL1mol/L��NaOH��Һ��ϣ���Һ�Լ��ԣ�c��OH-��=$\frac{1��1-99��0.01}{100}$mol/L=10-4mol/L��pH=10���ʴ�Ϊ��4��10��
���� ���⿼���˵���ƽ�ⳣ��������ǿ���Ĺ�ϵ��ǿ����ʵĵ���ƽ�⼰��Ӱ���֪ʶ����Ŀ�Ѷ��еȣ�ע����ȷ������ʵĵ���ƽ�⼰��Ӱ�����أ���ȷ����ϵĶ����жϷ�����

| A�� | Ũ����������Һ�м�����Al+2OH-�TAlO2-+H2�� | |
| B�� | �Ȼ�������ˮ����ˮ��Al3++3H2O�TAl��OH��3+3H+ | |
| C�� | ������Һ�м�������������������ҺAl3++3OH-�TAl��OH��3�� | |
| D�� | ��������Һ�м����������������ҺAl3++4OH-�TAlO2-+2H2O |
��3H2S+2HNO3$\frac{\underline{\;��\;}}{\;}$3S��+2NO��+4H2O
��H2S+2HNO3$\frac{\underline{\;��\;}}{\;}$S��+2NO2��+2H2O
��4H2S+2HNO3$\frac{\underline{\;��\;}}{\;}$4S��+NH4NO3+3H2O
��5H2S+2HNO3�T5S��+N2��+6H2O
�����ѧ�й������֪ʶ���ж�����Ũ���ɴ�С��˳����ȷ���ǣ�������
| A�� | �٣��ڣ��ۣ��� | B�� | �ڣ��٣��ܣ��� | C�� | �ڣ��٣��ۣ��� | D�� | �ܣ��ۣ��٣��� |
| A�� | ��״���£�HFΪҺ̬������Ϊ��������ȶ� | |
| B�� | N�������5�����ӣ����Ը����ϼ�ֻ��-3�� | |
| C�� | ����ദ�ڽ�����ǽ����Ĺ���λ�ã������������뵼����� | |
| D�� | Cl-��S2-��Ca2+��K+�뾶��С |
| A�� | CH4���ӵı���ģ�ͣ�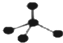 | B�� | NH4Cl�ĵ���ʽ��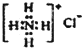 | ||
| C�� | S2-�ṹʾ��ͼ��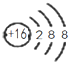 | D�� | �۱�ϩ�Ľṹ��ʽΪ��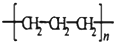 |
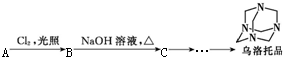
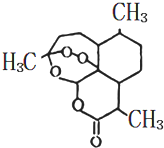 �ҹ���ѧ�����������ֲ��ɹ���ȡ�������أ�һ������ű����ҩ������2015��ŵ��������ѧ��ҽѧ���������ؽṹ��ʽ��ͼ���ṹ���й���������H2O2�����ƵĻ�ѧ���ʣ������й��������ص�˵������ȷ���ǣ�������
�ҹ���ѧ�����������ֲ��ɹ���ȡ�������أ�һ������ű����ҩ������2015��ŵ��������ѧ��ҽѧ���������ؽṹ��ʽ��ͼ���ṹ���й���������H2O2�����ƵĻ�ѧ���ʣ������й��������ص�˵������ȷ���ǣ�������| A�� | �����л��� | |
| B�� | �����ؾ���һ���������� | |
| C�� | �����ػ�ѧʽΪC15H20O5 | |
| D�� | ��������һ��������������NaOH��Һ��Ӧ |
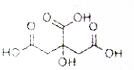 ����X�Ľṹʽ��ͼ��ʾ�������������ϻ���Ϊ���ϵ��ữ������ʳƷ��ҽѧ������������ϼ���Ҳ�ǻ�ѧ�м��壮���й�������X��˵����ȷ���ǣ�������
����X�Ľṹʽ��ͼ��ʾ�������������ϻ���Ϊ���ϵ��ữ������ʳƷ��ҽѧ������������ϼ���Ҳ�ǻ�ѧ�м��壮���й�������X��˵����ȷ���ǣ�������| A�� | X����ʽC6H7O7 | |
| B�� | 1mol����X���Ժ�3mol���������ӳ� | |
| C�� | X���Է���������Ӧ��ȡ����Ӧ����ȥ��Ӧ | |
| D�� | 1molX�ֱ���������NaHCO3��Na��Ӧ�õ������������Ϊ3��2 |
 NH4++NH2-��
NH4++NH2-��