��Ŀ����
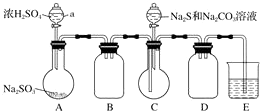
9��ʵ����������������Ҫ����������1������һ��������Ũ������Ũ����Ļ���ᣬ���뷴Ӧ���У�
��2���������µĻ��������μ���һ�����ı����������Ͼ���
��3 ����50���϶ȵ�60���϶��·�����Ӧ��ֱ����Ӧ������
��4 ����ȥ�����ֲ�Ʒ����������ˮ��5%��NaOH��Һϴ�ӣ������������ˮϴ�ӣ�
��5 ��������ˮCaCl2 �����Ĵ��������������õ�����������
��д���пհף�
��1������һ������Ũ������Ũ��������ʱ������ע��������Ӧ��Ũ������ע��Ũ�����У���ʱ���衢��ȴ
��2���ڲ���3�У�Ϊ��ʹ��Ӧ��50���϶ȵ�60���϶��½��У����õķ�������ˮԡ����
��3������4��ϴ�ӣ������������Ӧʹ�õ������Ƿ�Һ©��
��4������4�дֲ�Ʒ��5%��NaOH ��Һϴ�ӵ�Ŀ���dz�ȥ��������ᡢNO2����������
��5��������������ɫ���ܶȱ�ˮ����С���
���� ��1��Ũ������Ũ�����Ϸų��������ȣ����ƻ���Ӧ��Ũ������ע��Ũ�����У���ʱ���衢��ȴ����ֹ�������ˣ�
��2��Ϊ��ʹ��Ӧ��50���϶ȵ�60���϶��½��У�����ʹ��ˮԡ���ȵķ�����
��3�����뻥�����ܵ�Һ̬����ȡ��Һ��������Ҫ�÷�Һ©����
��4���Ʊ�������ʱҪ�õ����ᡢ���ᣬ������5% NaOH��Һϴ�ӣ�
��5���������������������ʴ��⣮
��� �⣺��1��Ũ������Ũ�����Ϸų��������ȣ����ƻ���Ӧ��Ũ������ע��Ũ�����У���ʱ���衢��ȴ����ֹ�������ˣ�
�ʴ�Ϊ��Ӧ��Ũ������ע��Ũ�����У���ʱ���衢��ȴ��
��2��Ϊ��ʹ��Ӧ��50���϶ȵ�60���϶��½��У�����ʹ����ˮԡ���ȣ��ʴ�Ϊ����ˮԡ���ȣ�
��3������4��ϴ�ӣ�����������������ˮ�����Է�����������ɲ�ȡ��Һ��������Ҫ�÷�Һ©�����ʴ�Ϊ����Һ©����
��4���Ʊ�������ʱҪ�õ����ᡢ���ᣬŨ�����ֽܷ������������������������������������������ᡢNO2���������ʣ�Ҫ��ȥ��������ᡢNO2���������ʣ�������5% NaOH��Һϴ�ӣ�
�ʴ�Ϊ����ȥ��������ᡢNO2���������ʣ�
��5���������������������ʿ�֪������������ɫ���ܶȱ�ˮ��Һ�壬�ʴ�Ϊ����
���� ���⿼���л�ʵ�飬�漰����������ȡ�����ضԻ��������Ŀ��飬��һ�����ۺ��ԣ���Ŀ�Ѷ��еȣ�����ʱע��ʵ�����֪ʶ��������ã�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�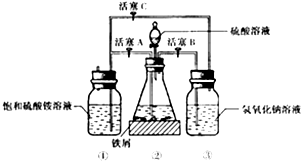 �����������һ��dz����ɫ���壬�׳�Ħ���Σ�
�����������һ��dz����ɫ���壬�׳�Ħ���Σ��仯ѧʽΪ��FeSO4•��NH4��2SO4•6H2O ���������ڿ������ױ����������γ�Ħ���κ���ȶ��ˣ���������刺�����������������淋����ʵ�������Ƶã������ε��ܽ�ȣ���λΪg/100gˮ�����±���
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 70 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 | 81.0 | 84.5 | 91.9 |
| FeSO4•7H2O | 40.0 | 48.0 | 60.0 | 73.3 | - | - |
| Ħ���� | 18.1 | 21.2 | 24.5 | 27.9 | 31.3 | 38.5 |
�ش��������⣺
��1������30%������������Һ��з���м�����������ۡ����⡢FeS�ȣ���������ˮϴ����������������Һ��е�Ŀ���dz�ȥ��м������
��2���������õ���м������ƿ�У�����ϡ���ᣬ��ƿ�з�����Ӧ�����ӷ���ʽ����ΪABCD������ţ�
A��Fe+2H+�TFe2++H2�� B��Fe2O3+6H+�T2Fe3++3H2O
C��2Fe3++H2S�T2Fe2++S��+2H+ D��2Fe3++Fe�T3Fe2+
��3�����������ڵķ�Ӧ������������ͨ��������Ӧ�رջ���A������BC������ĸ������������NaOH��Һ�������������������壬��ֹ��Ⱦ����������������ͨ��������Ŀ���Ƿ�ֹ�������ӱ���������
����ƿ�е���м�췴Ӧ��ʱ���رջ���B��C������A�����������������Ὣ��ƿ�е����������������ٲ���δ��Ӧ��ϡ���ᣩѹ�������������Һ�ĵײ����ڳ����·���һ��ʱ�䣬�Լ�ƿ�ײ����ᾧ����������泥������������������Һ��Ͼ��ܵõ���������茶��壬��ԭ������������淋��ܽ����С�����������з��벢�õ�������������茶���IJ��������ǹ��ˡ��þƾ�ϴ�ӡ�����
���Ƶõ���������茶������������м�������Fe3+��Ϊ�ⶨ������Fe2+�ĺ�������ȡһ������Ϊ20.0g����������茶�����Ʒ���Ƴ���Һ����0.5mo1/LKMnO4��Һ�ζ�������Һ��Fe2+ȫ����������MnO?4����ԭ��Mn2+ʱ����KMnO4��Һ���20.00mL���ζ�ʱ����KMnO4��Һװ����ʽ������ʽ���ʽ���ζ����У��жϷ�Ӧ����ζ��յ������Ϊ��Һ�ճ����Ϻ�ɫ������30s���䣻������FeSO4����������Ϊ38%��
| A�� | NaHCO3��Na2CO3�ֱ������ᷴӦ | B�� | NaOH��Cu��OH��2�ֱ������ᷴӦ | ||
| C�� | Ca��OH��2��CaCl2�ֱ���Na2CO3��Ӧ | D�� | Ba��NO3��2��Ba��OH��2�ֱ���ϡ���ᷴӦ |
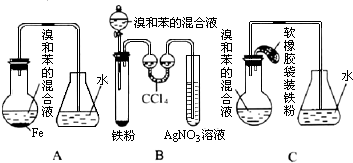
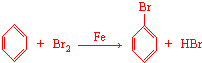 ��
��