��Ŀ����
17��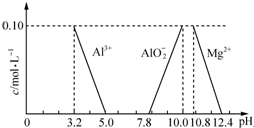 ��ϲ[AlaMgb��OH��c��CO3��d•xH2O]�������к�θ�ᣮ
��ϲ[AlaMgb��OH��c��CO3��d•xH2O]�������к�θ�ᣮ��1��1mol��ϲ��������������ȫ��Ӧ������ˮ�����ʵ���Ϊx+c+d���ú�x����ĸ�Ĵ���ʽ��ʾ����
��2����ϲ��ѧʽ��a��b��c��d�Ĺ�ϵΪa+b��c+d�����������=����������
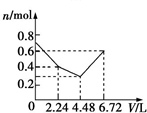
��3����֪�ڳ�������Һ��Al3+��Mg2+��Al${{O}_{2}}^{-}$��Ũ������ҺpH�Ĺ�ϵ��ͼ��ʾ��Ϊ�ⶨ��ϲ����ɣ�����������ʵ�飺
��ȡ��ϲ6.02g���飬��������2.00mol•L-1����ʹ���ܽ⣬����������85.00mLʱ��ʼ����CO2������������90.00mLʱ���÷�Ӧ��ȫ��
���ڢ�������Һ�м��������İ�ˮ�����ˮ�������������������Ȼ�þ�����Ե�����Һ��pH��5.0��7.0��ʹ�й�������ȫ������
�۽��ڲ�����ȫ���������ˡ�ϴ�ӣ����������أ�����Ϊ1.02g����ͨ������ȷ����ϲ�Ļ�ѧʽ��д��������̣���
���� ��1��1mol��ϲ�к�cmolOH-��dmolCO32-��xmolH2O�������ᷴӦ������ˮ��
��2������������ѭ����غ㣬��3a+2b=c+2d��
��3��ʵ��ټ���90mL���ᣬǡ����Һ��������Һ������Ϊ�Ȼ������Ȼ�þ��ʵ��ڼӰ�ˮʹAl3+ת��Ϊ��������ʱMg2+û��ת��Ϊ��������Һ������ΪNaCl��MgCl2����ʵ��ۿ�֪��1.02gΪ���������������Դ˼��㣮
��� �⣺��1��1mol��ϲ�к�cmolOH-��dmolCO32-��xmolH2O�������ᷴӦ������ˮ����ԭ���غ��֪������ˮ�����ʵ���Ϊ��x+c+d��mol���ʴ�Ϊ��x+c+d��
��2������������ѭ����غ㣬��3a+2b=c+2d�������Ӵ��ĵ�ɴ��������Ӵ��ĵ�ɣ��������ӵ���ĿС����a+b��c+d���ʴ�Ϊ������
��3������ͼ��֪��������Һ��pH��5.0��7.0��þ����û��ת��Ϊ������ֻ��������ת��Ϊ���������������Һ�м��������İ�ˮ����pH���ʴ�Ϊ����ˮ��
������������ʵ���Ϊ2.00mol•L-1��0.09L=0.18mol��
����CO2������������ʵ���Ϊ��0.09-0.085��L��2.00 mol•L-1��2=0.02mol��
CO32-�����ʵ���Ϊ0.02mol��2=0.01 mol��
�ɢٿ�֪������������к͵�OH-��Ȼ��CO32ת���HCO3-����������������HCO3-��Ӧ����CO2����n��Cl-��=n��OH-��+2n��CO32-������n��OH-��=0.18mol-0.01mol��2=0.16mol��
n��Al3+��=2n��Al2O3��=2��$\frac{1.02g}{102g/mol}$=0.02mol��
�ɵ���غ�2n��Mg2+��+3n��Al3+��=n��OH-��+2n��CO32-������2n��Mg2+��=0.16mol+0.01mol��2-0.02mol��3=0.12mol����n��Mg2+��=0.06mol��
m��H2O��=6.02g-0.6g-2.72g-1.44g-0.54g=0.72g����n��H2O��=$\frac{0.72g}{18g/mol}$=0.04 mol��
��n��Al3+����n��Mg2+����n��OH-����n��CO32-����n��H2O��=0.02��0.06��0.16��0.01��0.04=2��6��16��1��4����ϲ�����ΪAl2Mg6��OH��16CO3•4H2O��
�𣺴�ϲ�����ΪAl2Mg6��OH��16CO3•4H2O��
���� ���⿼�黯ѧ��Ӧ���йؼ��㣬Ϊ��Ƶ���㣬ע�������غ���м����ǽ��Ĺؼ���ע�������غ�˼�룬���ط�������������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�| A�� | ��ȥ���������۹�����ѡ���ȵ��ռ���Һ | |
| B�� | ��������������ҽ�þƾ���ʳ�ף�������ˮ | |
| C�� | Ѥ���ͷ��̻����������˼ء��ơ��ơ�ͭ�Ƚ���Ԫ�ص���ɫ��Ӧ�γɵ� | |
| D�� | ���ƿ���ȼ�ϵ�����������ͻ�����β����Ⱦ��ij�̶ֳ��Ͽ��Լ���PM2.5��Ⱦ |
��1����ˮ�к������ѧ���ʣ�����ֱ�����ã����Խ���ˮת��Ϊ��ˮ��һ���ش���п��⣮���������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ��
 ��������������ˮʱ�����ҿɻ�õ���Ҫ����ԭ�����������������ƣ�
��������������ˮʱ�����ҿɻ�õ���Ҫ����ԭ�����������������ƣ���2����ˮ�к��д�����NaCl���������Ŀǰ��ˮ���ε���Ҫ�����������Ϊ��ˮ�ء������غͽᾧ�أ������������BC����������д��ţ���
A��ѡ���뽭���뺣�ڱȽϽ��ĵط�B���������C����ϫ��������ƽ̹�տ��ĺ�̲
��3������������Ϊ���Σ������ô������ռ�������ʳ��ˮ�������ξ��ƣ���һ�ξ�����Ҫ���ó�������ȥ����ˮ�е�Ca2+��Mg2+��Fe3+��SO42-�����ӣ��������£�
����ˮ$\stackrel{����BaCl_{2}��Һ}{��}$$\stackrel{����Na_{2}CO_{3}��Һ}{��}$$\stackrel{����NaOH��Һ}{��}$$\stackrel{����}{��}$��Һ$��_{��pH}^{����}$��һ�ξ���ʳ��ˮ
��֪��20��C���ֳ������ܽ�ȣ�g�����±���
| CaSO4 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
�����ñ������ݽ�����Iѡ��BaCl2����ѡ��CaCl2��ԭ��BaSO4���ܽ�ȱ�CaSO4�ĸ�С���ɽ�SO42-�����ĸ���ȫ��
��4����ҵ��ͨ����NaCl��CO2 �� NH3 Ϊԭ����ȡ�����д����һ����ȡNaHCO3�Ļ�ѧ����ʽNaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl��
��5����ҵ�ƵõĴ������NaCl���ʣ��������������Բⶨ��Ʒ��NaCl��������������Ʒm��$��_{�ܽ�}^{H_{2}O}$��Һ$��_{����}^{����BaCl_{2}��Һ}$����$��_{ϴ��}^{H_{2}O}$$\stackrel{���º�ɡ���ȴ������}{��}$����n��
�ټ�������Ƿ�ϴ�Ӹɾ��ķ�����ȡ���һ��ϴ��Һ���Թ��У�����ϡH2SO4��Һ����AgNO3��HNO3����Һ������������ɫ�����������û��ϴ�Ӹɾ������ް�ɫ�������������ϴ�Ӹɾ���
����Ʒ��NaCl������������ѧ����ʽΪ��1-$\frac{106n}{197m}$����100%��
| A�� | ��⾫��ͭʱ�����ҺCuSO4��Һ�����ʵ���Ũ�Ȳ��� | |
| B�� | NaClO��Һ��ͨ������CO2��ClO-ˮ��̶�������Һ������ǿ | |
| C�� | SO3��g��+H2O��l���TH2SO4��aq���ڳ��������Է����У���÷�Ӧ�ġ�H��0 | |
| D�� | 0.1 mo1•L-1 CH3COOH��Һ��ˮϡ�ͺ���Һ��$\frac{{c��CH}_{3}C{OO}^{-1}��}{{c��CH}_{3}COOH��}$��ֵ��С |
| A�� | ԭ�Ӱ뾶��С�ıȽϣ�r��X����r��Y����r��Z�� | |
| B�� | Ԫ��Y��Z�ļ����ӵĵ��Ӳ�ṹ��ͬ | |
| C�� | Ԫ��X�ļ���̬�⻯������ȶ��Ա�Ԫ��Y��ǿ | |
| D�� | ֻ��W��X��Y����Ԫ�صĻ������У������ӻ����Ҳ�й��ۻ����� |

 �����仯����Ӧ�ù㷺
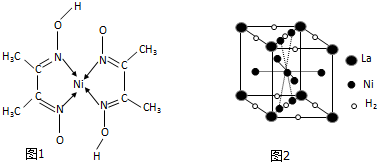
�����仯����Ӧ�ù㷺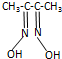 �� �����ڼ���Ni����һ�������£�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ��ͼ1��ʾ��
�� �����ڼ���Ni����һ�������£�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ��ͼ1��ʾ��