��Ŀ����
����Ŀ����.��ҵ�����̿���Ҫ�ɷ���MnO2������SiO2��Fe2O3���������ʣ�Ϊ��Ҫԭ���Ʊ������ܵĴ��Բ���̼���̣�MnCO3�����乤ҵ�������£�
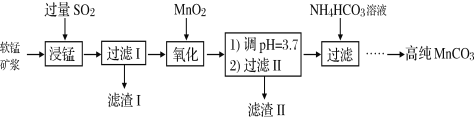
(1)���̹�����Fe2O3��SO2��Ӧ�����ӷ���ʽΪFe2O3+SO2+2H+=2Fe2++SO42-+H2O���÷�Ӧ�Ǿ�������������Ӧʵ�ֵġ�
����Fe2O3+6H+ =2Fe3++3H2O
����____________________________________________�����ӷ���ʽ����
(2)���ˢ�������Һ����Ҫ���ڵ����ֽ���������Ϊ____________�������ӷ��ţ���
(3)���������б�MnO2�����������У�д��ѧʽ����____________________��
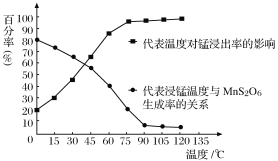
(4) ����������Ӧ�������и�����MnS2O6���ɣ��¶ȶ�����������Ӧ��Ӱ����ͼ��ʾ��Ϊ����MnS2O6�����ɣ����������������¶���_________������ˢ����õ���Һ�м���NH4HCO3��Һʱ�¶Ȳ���̫�ߵ�ԭ����______________________��
(5).����NH4HCO3��Һ������MnCO3������ͬʱ�����������ɣ�д����Ӧ�����ӷ���ʽ��_______________________________________________________��
��.�ؽ���Ԫ�ظ��Ķ��Խϴ�����ˮ�辭������������ŷš�
���������£����۸���Ҫ��Cr2O72-��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O72-�ķ�ˮ��ʵ��������ͼװ��ģ�����Cr2O72-��ˮ��������ӦʽFe-2e-=Fe2+��������Ӧʽ2H++2e-=H2�����ŵ����У���������pH���ߡ�
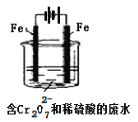
(1)���ʱ�ܷ���Cu�缫������Fe�缫?____(������������������)��������______________________��
(2)���ʱ����������Һ��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ______________________________��
(3)������Ӧ�õ��Ľ������������������ɳ�����ȫ�������ˮ�ĵ���ƽ��Ӱ��ǶȽ�����ԭ��__________________________��
���𰸡�2Fe3++SO2 +2H2O = 2Fe2++SO42��+4H+ Fe2+��Mn2+ Fe2+��SO2 90�� ��ֹNH4HCO3���ȷֽ⣬���ԭ�������� Mn2++2HCO3��=MnCO3��+CO2��+H2O ���� ������������Cu2+����ʹCr2O72��ԭ���ͼ�̬ 6Fe2++Cr2O72+14H+ = 6Fe3++2Cr3++7H2O ˮ�е�H+���������ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ��H2OH++OH�����ƶ���������OHŨ���������������������������϶�������ȫ
��������
��(1)�����ܷ�Ӧ��ȥ��������Ӧ��2Fe3++SO2 +2H2O = 2Fe2++SO42��+4H+��
(2)���̹����ж������̺Ͷ���������������ԭ��Ӧ����˹�����������Һ����Ҫ���ڵ����ֽ���������ΪFe2+��Mn2+��
(3)���������б�MnO2������������Ҫ�ǹ����Ķ��������Fe2+��
(4)����ͼ��֪��90��ʱ��MnS2O6�������ʵͣ��̽����ʸߣ��¶��ٸߣ��仯���������������������¶���90�棬NH4HCO3�����ֽ⣬�¶Ȳ���̫�ߣ���ֹNH4HCO3���ȷֽ⣬���ԭ�������ʡ�
(5)����NH4HCO3��Һ��������˫ˮ�⣬����MnCO3������ͬʱ�����������ɡ�
��(1)ͭʧȥ���ӵõ�����������ͭ���ӣ������Ӳ����л�ԭ�ԣ����ܺ��ظ����֮�䷢����Ӧ��
(2)Cr2O72������ǿ�����ԣ����Խ�������������Ϊ�����ӣ���������ԭΪ2Cr3+�����д�����ӷ���ʽ��
(3)ˮ�е�H+���������ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ��H2OH++OH�����ƶ���������OH��
��(1)�����ܷ�Ӧ��ȥ��������Ӧ��2Fe3++SO2 +2H2O = 2Fe2++SO42��+4H+���ʴ�Ϊ��2Fe3++ SO2 + 2H2O = 2Fe2++SO42��+4H+��
(2)���̹����ж������̺Ͷ���������������ԭ��Ӧ����˹�����������Һ����Ҫ���ڵ����ֽ���������ΪFe2+��Mn2+���ʴ�Ϊ��Fe2+��Mn2+��
(3)���������б�MnO2������������Ҫ�ǹ����Ķ�������ͽ��̹��������ɵ�Fe2+���ʴ�Ϊ��Fe2+��SO2��
(4)����ͼ��֪��90��ʱ��MnS2O6�������ʵͣ��̽����ʸߣ��¶��ٸߣ��仯���������������������¶���90�棬NH4HCO3�����ֽ⣬�¶Ȳ���̫�ߣ���ֹNH4HCO3���ȷֽ⣬���ԭ�������ʣ��ʴ�Ϊ��90�棻��ֹNH4HCO3���ȷֽ⣬���ԭ�������ʡ�
(5)����NH4HCO3��Һ��������˫ˮ�⣬����MnCO3������ͬʱ�����������ɣ�д����Ӧ�����ӷ���ʽ��Mn2++2HCO3��=MnCO3��+CO2��+H2O���ʴ�Ϊ��Mn2++2HCO3��= MnCO3��+CO2��+H2O��
��(1)ͭʧȥ���ӵõ�����������ͭ���ӣ������Ӳ����л�ԭ�ԣ����ܺ��ظ����֮�䷢����Ӧ���ʴ�Ϊ�����ܣ�������������Cu2+����ʹCr2O72��ԭ���ͼ�̬��
(2)Cr2O72������ǿ�����ԣ����Խ�������������Ϊ�����ӣ���������ԭΪ2Cr3+��������ӷ���ʽΪ6Fe2++Cr2O72+14H+ = 6Fe3++2Cr3++7H2O���ʴ�Ϊ��6Fe2++Cr2O72+14H+= 6Fe3+ + 2Cr3++7H2O��
(3)ˮ�е�H+���������ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ��H2OH++OH�����ƶ���������OH���ʴ�Ϊ��ˮ�е�H+���������ŵ磬H+Ũ�ȼ�С��ʹˮ�ĵ���ƽ��H2OH++OH�����ƶ���������OHŨ���������������������������϶�������ȫ��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ����ʽ̼��ͭ[Cu2(OH)2CO3]��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Դ�ͭмΪԭ����ȡ����������:

(1)���ܽ⡱���跴Ӧ�����ӷ���ʽΪ_______���¶��˿�����50�����ң�������50������Һ��O2�ܽ������١�Cu��ת���ʽ��ͣ�������50�� ��_____
(2)����Ӧ����������Cu2(OH)2CO3�Ļ�ѧ����ʽΪ_________����Ӧ����Һ��ͭԪ�ز�������pH�ͷ�Ӧʱ���Ӱ����ͼ��ʾ:

�жϷ�Ӧ���������: pHΪ___����Ӧʱ��Ϊ____h��
(3)����Cu2(OH)2CO3ϴ���Ƿ���ȫ�ķ�����____________________
(4) Cu2(OH)2CO3Ҳ���Ա�ʾΪCuCO3��Cu(OH)2�� �������ף�������Ӧ�����»�������������CuCO3��Cu(OH)2��Ϊ�ⶨ��Ʒ�Ĵ���[��Ʒ��Cu2(OH)2CO3����������]��ȡ10.97g������Ʒ��400�����Ҽ��ȣ���ù���������ʱ��仯��ϵ��ͼ��ʾ��

��֪: 8.00g����Ϊ��ɫ�����
�й����ʵ�Ħ�����������
���� | CuCO3��Cu(OH)2 | CuCO3��2Cu(OH)2 | CuO |
Ħ������/g��mol-1 | 222 | 320 | 80 |
������Ʒ�Ĵ���(д��������̣��������3λ��Ч����)��______

