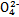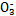��Ŀ����
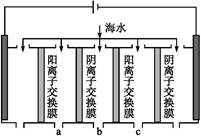
����������й�������Һ���ʱ�ı仯�����
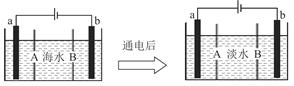
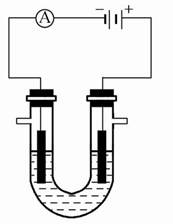
(1)��ʯī���缫������ͼװ�õ��AlCl3��Һ���������������ݣ��������г������ɡ�������⣬��������������Һ�л��ɹ۲쵽�������� �����ʹ���������ӷ���ʽ�� ��
(2)����ʯī���缫���NaCl��Al2(SO4)3�Ļ����Һ�������Һ�ж��ߵ����ʵ���Ũ�ȷֱ�Ϊ3 mol��L-1��0.5 mol��L-1�������б�ʾ�����̵�������ȷ���� ��
(1)��ʯī���缫������ͼװ�õ��AlCl3��Һ���������������ݣ��������г������ɡ�������⣬��������������Һ�л��ɹ۲쵽�������� �����ʹ���������ӷ���ʽ�� ��

(2)����ʯī���缫���NaCl��Al2(SO4)3�Ļ����Һ�������Һ�ж��ߵ����ʵ���Ũ�ȷֱ�Ϊ3 mol��L-1��0.5 mol��L-1�������б�ʾ�����̵�������ȷ���� ��

(1)��ɫ�������ܽ�����ʧ Al(OH)3+OH-=AlO2-+2H2O
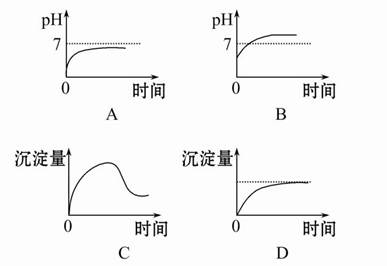
(2)A��D
(2)A��D
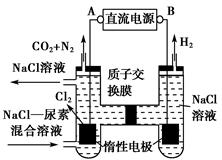
(1)�ö��Ե缫���AlCl3��Һ����ʼʱ�ܷ�Ӧ����ʽ�ɱ�ʾΪ2AlCl3+6H2O  2Al(OH)3��+3Cl2��+3H2��������������ΪAl(OH)3��H2�����������а�ɫ����Al(OH) 3���ɣ����ŵ��Ľ��У�AlCl3�����꣬��ʱ�൱�ڵ��ˮ��������H+�ŵ磬����������������ǿ��֮ǰ���ɵ�Al(OH)3�������ܽ⣬���ӷ���ʽ�ɱ�ʾΪAl(OH)3+OH-=AlO2-+2H2O��
2Al(OH)3��+3Cl2��+3H2��������������ΪAl(OH)3��H2�����������а�ɫ����Al(OH) 3���ɣ����ŵ��Ľ��У�AlCl3�����꣬��ʱ�൱�ڵ��ˮ��������H+�ŵ磬����������������ǿ��֮ǰ���ɵ�Al(OH)3�������ܽ⣬���ӷ���ʽ�ɱ�ʾΪAl(OH)3+OH-=AlO2-+2H2O��
(2)�����ǶԻ����Һ�ĵ�⣬�ɶ�4�����ӽ���������ϣ�����֪�����NaCl��Al2(SO4)3�Ļ����Һ�൱�ڵ��1 mol��L-1 AlCl3��Һ�����������ܷ�Ӧʽ�ɱ�ʾΪ2AlCl3+6H2O 2Al(OH)3��+3H2��+3Cl2�����ɴ˿�֪ѡ��A��D��ȷ��
2Al(OH)3��+3H2��+3Cl2�����ɴ˿�֪ѡ��A��D��ȷ��
 2Al(OH)3��+3Cl2��+3H2��������������ΪAl(OH)3��H2�����������а�ɫ����Al(OH) 3���ɣ����ŵ��Ľ��У�AlCl3�����꣬��ʱ�൱�ڵ��ˮ��������H+�ŵ磬����������������ǿ��֮ǰ���ɵ�Al(OH)3�������ܽ⣬���ӷ���ʽ�ɱ�ʾΪAl(OH)3+OH-=AlO2-+2H2O��
2Al(OH)3��+3Cl2��+3H2��������������ΪAl(OH)3��H2�����������а�ɫ����Al(OH) 3���ɣ����ŵ��Ľ��У�AlCl3�����꣬��ʱ�൱�ڵ��ˮ��������H+�ŵ磬����������������ǿ��֮ǰ���ɵ�Al(OH)3�������ܽ⣬���ӷ���ʽ�ɱ�ʾΪAl(OH)3+OH-=AlO2-+2H2O��(2)�����ǶԻ����Һ�ĵ�⣬�ɶ�4�����ӽ���������ϣ�����֪�����NaCl��Al2(SO4)3�Ļ����Һ�൱�ڵ��1 mol��L-1 AlCl3��Һ�����������ܷ�Ӧʽ�ɱ�ʾΪ2AlCl3+6H2O
 2Al(OH)3��+3H2��+3Cl2�����ɴ˿�֪ѡ��A��D��ȷ��
2Al(OH)3��+3H2��+3Cl2�����ɴ˿�֪ѡ��A��D��ȷ��
��ϰ��ϵ�д�
 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
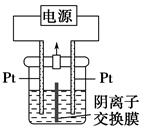
 KIO3��3H2��
KIO3��3H2�� 
 R2Cu(�л���)��2H��(ˮ��)
R2Cu(�л���)��2H��(ˮ��)