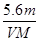��Ŀ����
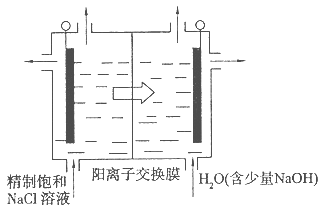
��ˮ�����ķ����ж��֣����������������ȡ�����������һ���������ӽ���Ĥ�������ӽ����ķ�������ԭ����ͼ��ʾ����֪��ˮ�к�Na����Cl����Ca2����Mg2����SO�����ӣ��缫Ϊ���Ե缫��������������ȷ���ǣ�������
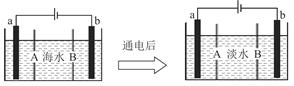

| A��BĤ�������ӽ���Ĥ |
| B��ͨ���ˮ����������a�缫���˶� |
| C��ͨ���a�缫�ĵ缫��ӦʽΪ4OH����4e��=O2����2H2������ |
| D��ͨ���b�缫�ϲ�����ɫ���壬��Һ�г���������ɫ���� |
D
����ͼ��ʾ��b�缫������ӵ�Դ�ĸ�����Ϊ���������ʱ���������������ƶ�����BĤӦΪ�����ӽ���Ĥ��A�����a�缫������ӵ�Դ��������Ϊ���������ʱ���������������ƶ���B�����Cl��ʧ��������ǿ��OH�����ʸõ���������ӦΪ2Cl����2e��=Cl2����C����õ���������ӦΪ2H2O��Mg2����2e��=H2����Mg��OH��2����D����ȷ��

��ϰ��ϵ�д�
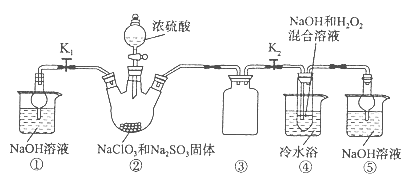
�����Ŀ
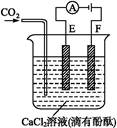

 H2������:4OH--4e-
H2������:4OH--4e-
 NA
NA