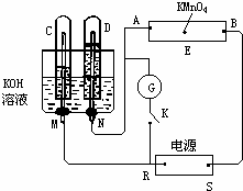
��Ŀ����
5��P��Q��W��X��Y��Z��Ԫ�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������Wԭ������������������������֮����3��4��P����W�γ����ֳ����Ļ�����M��N���������ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1��Q��Y���������ǵ����������Ҫ���ʣ�X�ǵؿ��к�����ߵĽ���Ԫ�أ�Z���γɺ�ɫ����ש��ɫ����Z2O�ͺ�ɫ��ZO�����������1��N�ĵ���ʽΪ��
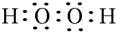 ��W��X��Y�����Ӱ뾶�ɴ�С��˳��ΪS2-��O2-��Al3+�������ӷ��Żش�
��W��X��Y�����Ӱ뾶�ɴ�С��˳��ΪS2-��O2-��Al3+�������ӷ��Żش���2��XQ��һ�����͵Ľṹ�մɲ��ϣ��߱��������������ܣ��ϳɵķ���֮һ�Ǹ���ʱ�ù�����̼��Q��������ķ�Χ�л�ԭX���������д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��N2+Al2O3+3C$\frac{\underline{\;����\;}}{\;}$2AlN+3CO
��3����֪����ʱNaPYW3����Һ��Һ���ֽ�ǿ�����ԣ�������Һ�и�����Ũ���ɴ�С������˳��Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-��
��4��Q���⻯����Y������������Ӧ��ˮ���ﷴӦ����һ�����Σ���ˮ��Һ�����ԣ�ԭ����NH4++H2O?NH3•H2O+H+�����ӷ���ʽ���ͣ�
��5��ZCl2��Һ�л���FeCl3����ʱ���ɼ���CuO�����Լ���ѧʽ������pH=4���ٹ��ˣ���Fe��OH��3��KSP=10-35����ѧʽ��Ϊ��������Һ�е�����Ũ��С��10-5mol/Lʱ�����ʹ���ȫ����
��6����ҵ�Ͽ��ø���������O2+Z2Y�T2Z+YO2��ұ������Z������1molZʱת�Ƶĵ�������Ϊ3mol��
���� P��Q��W��X��Y��Z��Ԫ�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������Wԭ������������������������֮��Ϊ3��4����WΪOԪ�أ�P����W�γ����ֳ����Ļ�����M��N���������ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1����PΪHԪ�أ�MΪH2O��NΪHO2O2��Q��Y���������ǵ����������Ҫ���ʣ��������������Ҫ�����Ƕ��������Ͷ�������Yԭ����������Q����QΪNԪ�أ�YΪSԪ�أ�X�ǵؿ��к�����ߵĽ���Ԫ�أ���X��AlԪ�أ�Z���γɺ�ɫ����ש��ɫ����Z2O�ͺ�ɫ��ZO�����������Z��CuԪ�أ��γɵ�����������ΪCu2O��ש��ɫ����CuO����ɫ�����ݴ˽��
��� �⣺P��Q��W��X��Y��Z��Ԫ�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������Wԭ������������������������֮��Ϊ3��4����WΪOԪ�أ�P����W�γ����ֳ����Ļ�����M��N���������ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1����PΪHԪ�أ�MΪH2O��NΪHO2O2��Q��Y���������ǵ����������Ҫ���ʣ��������������Ҫ�����Ƕ��������Ͷ�������Yԭ����������Q����QΪNԪ�أ�YΪSԪ�أ�X�ǵؿ��к�����ߵĽ���Ԫ�أ���X��AlԪ�أ�Z���γɺ�ɫ����ש��ɫ����Z2O�ͺ�ɫ��ZO�����������Z��CuԪ�أ��γɵ�����������ΪCu2O��ש��ɫ����CuO����ɫ����
��1��NΪHO2O2������ʽΪ��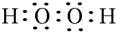 ��W��X��Y�����ӷֱ�ΪO2-��Al3+��S2-�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶�ɴ�С��˳��Ϊ��S2-��O2-��Al3+��
��W��X��Y�����ӷֱ�ΪO2-��Al3+��S2-�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶�ɴ�С��˳��Ϊ��S2-��O2-��Al3+��
�ʴ�Ϊ��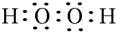 ��S2-��O2-��Al3+��
��S2-��O2-��Al3+��
��2������ʱ�ù�����̼�ڵ����ķ�Χ�л�ԭAl2O3�õ�AlN����Ӧ������CO���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��N2+Al2O3+3C$\frac{\underline{\;����\;}}{\;}$2AlN+3CO��
�ʴ�Ϊ��N2+Al2O3+3C$\frac{\underline{\;����\;}}{\;}$2AlN+3CO��
��3������ʱNaHSO3����Һ��Һ���ֽ�ǿ�����ԣ���Һ��c��H+����c��OH-����˵��HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ���Һ��������Դ��ˮ���HSO3-���룬����Һ�У�H+����c��SO32-����������ˮ��̶ȶ�������Һ��������Ũ��������Һ��c��HSO3-����c��H+��������������ˮ�ĵ������������Һ��c��SO32-����c��OH-��������Һ�и�����Ũ���ɴ�С������˳��Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��4��Q���⻯��ΪNH3��Y������������Ӧ��ˮ����ΪH2SO4����Ӧ����һ������Ϊ��NH4��2SO4����ˮ��Һ�д��ڣ�NH4++H2O?NH3•H2O+H+�������ԣ�
�ʴ�Ϊ���NH4++H2O?NH3•H2O+H+��
��5��CuCl2��Һ�л���FeCl3����ʱ���ɼ���CuO�ȵ���pH���ٹ��˳�ȥ����Һ�е�����Ũ��С��10-5mol/Lʱ�����ʹ���ȫ����10-35=c��Fe3+����c3��OH-������֪c��OH-��=10-10mol/L������ҺpH=-lg$\frac{1{0}^{-14}}{1{0}^{-10}}$=4��Ӧ����pH=4��
�ʴ�Ϊ��CuO��4��
��6����ҵ�Ͽ��ø���������O2+Cu2S�T2Cu+SO2��ұ������Cu����Ӧ��ֻ��SԪ�ػ��ϼ����ߣ���-2������Ϊ+4�ۣ�����1molCuʱ�õ���������Ϊ0.5mol��ת�Ƶĵ�������Ϊ0.5mol��6=3mol��
�ʴ�Ϊ��3��
���� ������Ҫ����Ԫ���ƶϡ�����ʽ�����뾶�Ƚϡ�����Ũ�ȴ�С�Ƚϡ�����ˮ�⡢����ת��Ӧ�á��ܶȻ��йؼ��㡢����ת����Ŀ����ȣ��ƶ�Ԫ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ�����������������������Ѷ��еȣ�

| A�� | �����¶� | B�� | ����B��Ũ�� | ||
| C�� | ����ѹǿ | D�� | ʹ�ú��ʵĴ��� |
| A�� | �����Ȼ������������������з�����ѧ�仯���� | |
| B�� | ��������������Һ�Ŀ�����ֱ����10-9��10-7m֮�� | |
| C�� | �����ö����ЧӦʵ����Ч�����Ȼ�����Һ������������Һ | |
| D�� | ���Ȼ�����������ˮ�� |
��1����Ԫ�������ڱ���λ�õ������ڵڢ�A�壬�����ӵĽṹʾ��ͼΪ
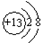 ��
����2��Fe3+��Al3+��Cu2+���ֽ�����������������ǿ����������˳����Fe3+��Cu2+��Al3+�������·��Ĺ����У�FeCl3��Һ����ʴ�̸ֲ����÷�Ӧ�����ӷ���ʽΪ2Fe3++Cu=2Fe2++Cu2+��
��3�����������ƣ�������Ҳ��������ˮ��������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ����Fe3++3H2O?Fe��OH��3�����壩+3H+�����Է�ˮ����Fe3+��ˮ�⣬ʹ�䲻���������������õ�Fe��OH��3���壮
��4���±��У��Գ��������ȷ�Ե��ж϶���ȷ����BC����д��ĸ��ţ���
| ѡ�� | ������ | ������ | �ж� |
| A | ���ǵؿ��к�����ߵĽ���Ԫ�� | ������������ʹ�õĽ������� | ��ԣ���ԣ� |
| B | �����ھƾ��ƻ����ϼ����ڻ��������� | �������ۻ������н�ǿ���������� | ��ԣ������ |
| C | ���ۿ��Ժ���������ĩ�������ȷ�Ӧ | ���ȷ�Ӧ�ǹ�ҵ������÷��� | ��ԣ������ |
| D | ͭ�ڳ�ʪ�����б��������ͭ�� | ����ͭ����ʢ��Ũ���� | ��ԣ���ԣ� |
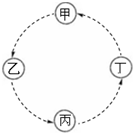 �ס��ҡ����������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�P��Ӧ��������ȥ����ͷ��ʾһ��ת���������и��������У���������ͼʾת����ϵ���ǣ�������
�ס��ҡ����������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�P��Ӧ��������ȥ����ͷ��ʾһ��ת���������и��������У���������ͼʾת����ϵ���ǣ�������| �� | �� | �� | �� | |
| A | Fe | FeCl3 | Fe ��OH��3 | Fe2O3 |
| B | NaAlO2 | Al ��OH��3 | Al2O3 | Al |
| C | Na | Na2O2 | Na2CO3 | NaCl |
| D | SiO2 | H2SiO3 | Na2SiO3 | Si |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 33.6L�������к��з�ԭ�ӵ���ĿΪ1.5NA | |
| B�� | ���³�ѹ�£�7.0g��ϩ���ϡ�Ļ�����к�����ԭ�ӵ���ĿΪNA | |
| C�� | 50mL18.4 mol/LŨ����������ͭ�ȷ�Ӧ������SO2���ӵ���ĿΪ0.46NA | |
| D�� | ij�ܱ�����ʢ��0.1molN2��0.3molH2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0.6NA |
| A�� | ��һ�������£�ʹú���������ã����Եõ�Һ��ȼ�ϣ�Ҳ���Ի�ýྻ��ȼ���� | |
| B�� | ��CH3��2CHCH��CH3��2��ϵͳ����Ϊ��2��3-�������� | |
| C�� | Ӳ֬�������������ϡ������ˮ�⣬Ҳ������NaOH��Һ�з���������Ӧ����ˮ�� | |
| D�� | ����������о����а������Ȼ�������Ũ����������Һ�ɴ���Һ������ |