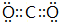��Ŀ����
11��A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ����������������֪��AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������Ӧ��ˮ���������⻯�ﷴӦ�������Σ�D��Eͬ���壬EԪ��ԭ�ӵ������������ȴ�����������2����ش���1��Ԫ��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壮
��2��Ԫ��D�ĵ���������Ʒ�Ӧ���ɵĻ�������DZˮ����еĹ����������ֻ�������ˮ��Ӧ�����ӷ���ʽ2Na2O2 +2H2O=4Na++4OH-+O2����
��3��D��E��Ԫ����Ƚϣ���ԭ�ӵõ���������ǿ��������д���ƣ�������˵���У�����֤���������۵���bc����д��ţ�
a���Ƚ�������Ԫ�صij������ʵķе� b�������γɵĻ������У�DԪ�ص�ԭ���Ը���
c���Ƚ�������Ԫ�ص���̬�⻯����ȶ��� d���Ƚ�������Ԫ���⻯���ˮ��Һ������
��4��Ԫ��A��C��D����Ԫ�ؿ��γ�һ���Σ���ˮ��Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��NH4++H2O?NH3��H2O+H+��
��5����Ԫ��A��B��D��ɵ�һԪ��XΪ�ճ������еĵ�ζ����Ԫ��A��F��ɵĻ�����ΪY���ڵ��������pH��X��Y����Һ�зֱ�����������п�ۣ���Ӧ����������һ����Һ�д���п�ۣ���Ӧ����������Һ�з�Ӧ���ʵĴ�С��ϵ�ǣ�X��Y�����������=����������
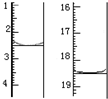
��6��Ԫ��F�ĵ��ʳ�������һ�����壬��ҵ����Ҫ��ͨ����������εı�����Һ�ķ�����ø����壬�ٶ�װ��ı�����ҺΪ100mL�����ǰ����Һ����仯�ɺ��ԣ�������������ϲ���11.2mL����״��������ʱֹͣͨ�磬����Һҡ�ȣ���ʱ��Һ��pHΪ12��
���� A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶��С����AΪHԪ�أ�
BԪ��ԭ�ӵ��������������ڲ��������2������BΪCԪ�أ�CԪ�ص�����������Ӧ��ˮ���������⻯�ﷴӦ�������Σ���CΪNԪ�أ�D��Eͬ���壬EԪ��ԭ�ӵ������������ȴ�����������2����DΪOԪ�أ�EΪSԪ�أ���ΪFΪ������Ԫ�أ�ԭ����������S������FΪClԪ�أ��Դ˽����⣮
��� �⣺A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ�Ӱ뾶��С����AΪ��Ԫ�أ�BԪ��ԭ�ӵ��������������ڲ��������2������BΪ̼Ԫ�أ�CԪ�ص�����������Ӧ��ˮ���������⻯�ﷴӦ�������Σ���CΪ��Ԫ�أ�D��Eͬ���壬EԪ��ԭ�ӵ������������ȴ�����������2����DΪ��Ԫ�أ�EΪ��Ԫ�أ���ΪFΪ������Ԫ�أ�ԭ����������������FΪ��Ԫ�أ�
��1��CΪ��Ԫ�أ������ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2��Ԫ��D�ĵ���������Ʒ�Ӧ���ɵĻ��������DZˮ����еĹ�����������ΪNa2O2������������ˮ��Ӧ�����ӷ���ʽΪ��2Na2O2 +2H2 O=4Na++4OH-+O2����
�ʴ�Ϊ��2Na2O2 +2H2 O=4Na++4OH-+O2����
��3��ͬ�������϶��·ǽ����Լ������ʷǽ�����O��S��OԪ�صõ���������ǿ��
a���е������������ʣ�����˵���õ�������ǿ������a����
b�������γɵĻ������У�OԪ�ص�ԭ���Ը��ۣ�˵��O�������ӵ�����ǿ����b��ȷ��
c���⻯��Խ�ȶ�������Ԫ�صķǽ�����Խǿ���õ�������ǿ����c��ȷ��
d���⻯���ˮ��Һ����ǿ��������˵������Ԫ�صĵõ�������ǿ������HFΪ���ᡢHClΪǿ�ᣬ��Fԭ�ӵõ���������ǿ����d����
�ʴ�Ϊ������bc��
��4��A��C��D���γ�һ����ΪNH4NO3����Һ笠�����ˮ�⣺NH4++H2O?NH3��H2O+H+����ˮ��Һ�����ԣ�
�ʴ�Ϊ��NH4++H2O?NH3��H2O+H+��
��5����Ԫ��H��C��O��ɵ�һԪ��XΪ�ճ������еĵ�ζ������XΪ���ᣬԪ��H��Cl��ɵĻ�����ΪHCl�����������ᡢ������ǿ�ᣬ�淴Ӧ���д�����Լ�������������ӣ���Zn��Ӧ�����ʸ��죬�ʷ�Ӧ����������Һ�з�Ӧ���ʵĴ�С��ϵ�ǣ�X��Y��
�ʴ�Ϊ������
��6����ⱥ��ʳ��ˮ�������������������ʵ���Ϊ$\frac{0.0112L}{22.4L/mol}$=0.0005mol����2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2����֪��n��NaOH��=0.0005mol��2=0.001mol����c��OH-��=$\frac{0.001mol}{0.1L}$=0.01mol/L��c��H+��=$\frac{1{0}^{-14}}{1{0}^{-2}}$mol/L=10-12mol/L����pH=-lg10-12=12��
�ʴ�Ϊ��12��
���� ���⿼��λ�ýṹ���ʹ�ϵ�����û�ѧ���Ӱ�췴Ӧ���ʵ����ء����ԭ������ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | Al3+ Na+ NO3- Cl- | B�� | K+ Na+ Cl- NO3- | ||
| C�� | K+ Na+ Cl- AlO2- | D�� | K+ NH4+ SO42- NO3- |
��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3S2������S��Ԫ�أ�Ԫ�ط���ΪMg��
| A�� | ��HF��HCl��HBr��HI�����Ե�������ʵ���Ƴ�F��Cl��Br��I�ķǽ����Ե����Ĺ��� | |
| B�� | N�ķǽ����Ա�Pǿ��N2�Ļ�Ա����ʵ�ǿ | |
| C�� | Li��Na��K��ԭ�Ӱ뾶�͵����ܶ���ԭ�����������Ӷ����� | |
| D�� | ��������¶�շ������ױ��ʣ��������ǵ���ʵ���Ƴ�O�ķǽ����Ա�Sǿ |
 A��B��C��D����Ԫ�صĺ˵������С��18��AԪ��ԭ�Ӻ���ֻ��1�����ӣ�B�ǵؿ��к�������Ԫ�أ�B��C���γ����ֻ�����CB��CB2��C���������������۾���ֵ��ȣ�CB�ж���CB2���������D+������Neԭ����ͬ�ĵ��Ӳ�ṹ��
A��B��C��D����Ԫ�صĺ˵������С��18��AԪ��ԭ�Ӻ���ֻ��1�����ӣ�B�ǵؿ��к�������Ԫ�أ�B��C���γ����ֻ�����CB��CB2��C���������������۾���ֵ��ȣ�CB�ж���CB2���������D+������Neԭ����ͬ�ĵ��Ӳ�ṹ��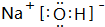 CB2�ĵ���ʽΪ
CB2�ĵ���ʽΪ