��Ŀ����
2������ˮ��IJ��C6H12O6�����������������Ʊ����ᣬװ����ͼ��ʾ�����ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
�ٽ�1��1�ĵ���ˮ��Һ���������ᣨ98%�������ձ��У�ˮԡ������85�桫90�棬����30min��Ȼ�����¶Ƚ���60�����ң�
�ڽ�һ�����ĵ���ˮ��Һ����������ƿ�У�
�ۿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ��ᣨ65%HNO3��98%H2SO4��������Ϊ2��1.5����Һ��
�ܷ�Ӧ3h���ң���ȴ����ѹ���˺�ò��ᾧ���Ʒ�����ؽᾧ�ò��ᾧ�壮������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6+12HNO3��3H2C2O4+9NO2��+3NO��+9H2O
C6H12O6+8HNO3��6CO2+8NO��+10H2O
3H2C2O4+2HNO3��6CO2+2NO��+4H2O
��ش��������⣺
��1��ʵ��ټ���98%����������Ŀ���ǣ��ӿ����ˮ����ٶȣ������������ã���
��2������ˮ�Ľ�����a����a��b����ʵ����������μӹ��죬�����²�������½�����ԭ�����¶ȹ��ߣ�����Ũ�ȹ�����H2C2O4��һ����������
��3��װ��B������������ȫƿ��Ϊʹβ��������գ�C���Լ���NaOH��Һ��
��4���ؽᾧʱ�������ᾧ���Ʒ���ټ����ܽ⡢�ڳ��ȹ��ˡ�����ȴ�ᾧ���ܹ���ϴ�Ӣݸ����ʵ�鲽�裬�õ��ϴ����IJ��ᾧ�壮�ù����пɽ���Ʒ���ܽ�Ƚϴ�������ڢܣ�������������ţ�ʱ��ȥ������Ʒ���ܽ�Ƚ�С�����������������ֽ�ϣ����ֽ�ϡ�����Һ�С�����
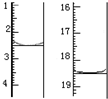
��5������Ʒ�ں�������Լ90�����º�������أ��õ���ˮ�ϲ��ᣮ��KMnO4����Һ�ζ����÷�Ӧ�����ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O����ȡ����Ʒ0.1200g��������ˮ��ȫ�ܽ⣬Ȼ����0.02000mol•L-1������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ����ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ84.0%��
���� ��1������Ũ������������Խ�Ϸ�Ӧ���
��2������Ч������Ч���ã�Ũ��������ˮ���ȣ�������л�ԭ�ԣ������ܽ�һ������C6H12O6��H2C2O4��
��3��װ��B�������Ƿ�ֹ����װ�ú�����װ�ü䷢����������Ӧ��β�����е������������Ⱦ��������Ҫ������������Һ���գ�
��4����������ʵ�鲽���֪��ͨ���ؽᾧ�ò��ᾧ��ʱ�����ᾧ���������ܽ�Ƚϴ������������Һ�У��ܽ�Ƚ�С������������ʱ������ֽ�ϣ�
��5�������������£�������������ܺͲ��ᷢ��������ԭ��Ӧ���ɶ��������ӡ�������̼��ˮ�����ݷ�Ӧ���㣮
��� �⣺��1��Ũ�������ǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�����ʵ���ǽ�C6H12O6���������������Ʊ����ᣬŨ������������Ũ������ˮ�����������ɲ���ķ����ƶ���
�ʴ�Ϊ���ӿ����ˮ����ٶȣ������������ã���
��2������Ч������Ч���ã�����ˮ�Ľ�����a��b��������Ϊ65%HNO3��98%H2SO4�Ļ��Һ�����Һ����ˮ���ȣ��¶ȸ��ܼӿ컯ѧ��Ӧ�������ܽ�һ������H2C2O4�ɶ�����̼��
�ʴ�Ϊ��a���¶ȹ��ߣ�����Ũ�ȹ�����H2C2O4��һ����������
��3��װ��B�������Ƿ�ֹ����װ�ú�����װ�ü䷢����������ȫƿ�����ã���Ӧ��β�����е������������Ⱦ��������Ҫ������������Һ���գ�����C���Լ���NaOH��Һ��
�ʴ�Ϊ������ȫƿ��NaOH��Һ��
��4����������ʵ�鲽���֪��ͨ���ؽᾧ�ò��ᾧ��ʱ�����ᾧ���������ܽ�Ƚϴ������������Һ�У�Ӧ���ڲ�����г�ȥ���ܽ�Ƚ�С������������ʱ������ֽ�ϣ�
�ʴ�Ϊ���ܣ���ֽ�ϣ�
��5�������������£�������������ܺͲ��ᷢ��������ԭ��Ӧ���ɶ��������ӡ�������̼��ˮ�����ӷ���ʽΪ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��ͼʾ�ζ���������Һ���=18.50mL-2.50mL=16.00mL��
n��KMnO4��=0.016L��0.0200mol•L-1=3.2��10-4mol�����ݷ���ʽ�ɵã�
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O
2 5
3.2��10-4mol 8��10-4mol
��Ʒ�ж�ˮ�ϲ��������Ϊm=8��10-4mol��126g/mol=8��126��10-4g=0.1008g��
��ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ$\frac{0.1008g}{0.12g}$��100%=84.0%��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��84.0%��
���� ������Ҫ�����˲������ȡʵ�飬ע�����ʵ���ԭ������������������ԭ�����ǽ��Ĺؼ���Ҫ��߱�һ�������۷��������ͼ������������������Ŀ�Ѷ��еȣ�

| A�� | �����������DZ����������� | |
| B�� | ������Ⱦ��Ҫ����������Ⱦ��ˮ��Ⱦ��������Ⱦ��ʳƷ��Ⱦ�� | |
| C�� | ������������ᷢ������������ | |
| D�� | ����������Ⱦ�ĸ������������ƻ�ѧ��ҵ�ķ�չ |
 �����ڶ��ǻ������������˵��������ȷ���ǣ�������
�����ڶ��ǻ������������˵��������ȷ���ǣ�������| A�� | ���ǻ�������Ⱦ�����������ʣ��־��зӵ����� | |
| B�� | 1mol���ǻ�������ֻ����1mol NaOH�����кͷ�Ӧ | |
| C�� | ���ǻ���������Һ�еμ����Ȼ�����Һ�����Եõ���ɫ��Һ | |
| D�� | ���ǻ��������ͨ���ױ���������ȡ����ˮ��ȷ�Ӧ�Ƶ� |
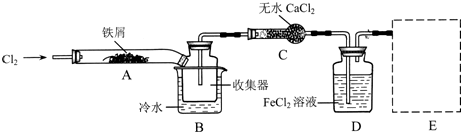
���������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�
�ټ���װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ��ɣ�
�ܡ�
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����N2�Ͼ�Cl2�����ռ����ܷ⣮
��ش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ2Fe+3Cl2 $\frac{\underline{\;\;��\;\;}}{\;}$2FeCl3
��2���ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A�Ҷˣ�Ҫʹ������FeCl3�����ռ������ڢܲ��������ڳ�����FeCl3�����·�����
��3�����������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ��ڢ�
��4��װ��B����ˮԡ������Ϊ��ȴ��ʹFeCl3�����������ռ���Ʒ��װ��C������Ϊ����ܣ�װ��D��FeCl2ȫ����Ӧ����ʧȥ����Cl2�����ö�ʧЧ��д������FeCl2�Ƿ�ʧЧ���Լ�������KMnO4��Һ��
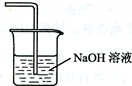
��5�������߿��л���β������װ��E��ע���Լ���
��6���ú���Al203��SiO2������FeO•xFe2O3�������Ʊ�Al2��SO4��3•18H2O�������������£����ֲ����������ԣ�
���������м������ϡH2SO4������
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��֪���������ӵ���ʼŨ��Ϊ0.1mol•L-1
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
| A�� | 0.5mol CO32- | B�� | 0.5mol Na+ | C�� | 3.01��1023��Na+ | D�� | 3.01��1023��O |
| A�� | H+��Mg2+��NO3-��Ba2+ | B�� | SO42-��Na+��HCO3-��K+ | ||
| C�� | NO3-��OH-��Cl-��Ba2+ | D�� | Cu2+��OH-��Fe2+��SO42- |