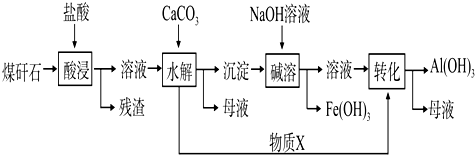
��Ŀ����
15��A��J��a��b�ֱ����ʮ�ֲ�ͬ���л������֮����ת����ϵ��ͼ��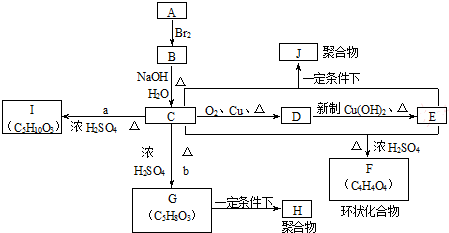
��1��A�ĽṹʽΪCH2=CH2��F�Ľṹ��ʽΪ
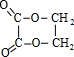 ��ָ��C��G�ķ�Ӧ����������ȡ������Ӧ��
��ָ��C��G�ķ�Ӧ����������ȡ������Ӧ����2��д������ת���Ļ�ѧ����ʽ
��C��E��JnCH2OHCH2CH2OH+nHOOCCOOH$\stackrel{����}{��}$
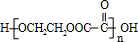 +��2n-1��H2O
+��2n-1��H2O��G��H
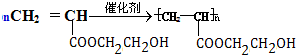
��3��д�����л���I��Ϊͬ���칹�壬�ҽṹ�к����Ȼ����ǻ����л��ﹲ12�֣��ֱ��ǣ�
���� C�������ᷴӦ������Cu������������������Ӧ��C�����ǻ�-OH����C��D��Eת����֪�����ߺ�����̼ͬԭ����Ŀ��C��E����F��F�ķ���ʽΪC4H4O4����C�к���2��Cԭ�ӣ���A��B��C��ת����֪��A�к���2��Cԭ�ӣ������巴Ӧ����AΪCH2=CH2��BΪBrCH2CH2Br��CΪHOCH2CH2OH��DΪOHC-CHO��EΪHOOC-COOH��FΪ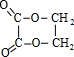 ��C��E�������۷�Ӧ��J���Ҷ�����b����������Ӧ����G�����G�ķ���ʽ��֪��bΪCH2=CHCOOH��GΪCH2=CHCOOCH2CH2OH���Ҷ�����a����������Ӧ����I������I�ķ���ʽ��֪��aΪCH3CH2COOH��IΪCH3CH2COOCH2CH2OH���ݴ˽��
��C��E�������۷�Ӧ��J���Ҷ�����b����������Ӧ����G�����G�ķ���ʽ��֪��bΪCH2=CHCOOH��GΪCH2=CHCOOCH2CH2OH���Ҷ�����a����������Ӧ����I������I�ķ���ʽ��֪��aΪCH3CH2COOH��IΪCH3CH2COOCH2CH2OH���ݴ˽��
��� �⣺C�������ᷴӦ������Cu������������������Ӧ��C�����ǻ�-OH����C��D��Eת����֪�����ߺ�����̼ͬԭ����Ŀ��C��E����F��F�ķ���ʽΪC4H4O4����C�к���2��Cԭ�ӣ���A��B��C��ת����֪��A�к���2��Cԭ�ӣ������巴Ӧ����AΪCH2=CH2��BΪBrCH2CH2Br��CΪHOCH2CH2OH��DΪOHC-CHO��EΪHOOC-COOH��FΪ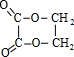 ��C��E�������۷�Ӧ��J���Ҷ�����b����������Ӧ����G�����G�ķ���ʽ��֪��bΪCH2=CHCOOH��GΪCH2=CHCOOCH2CH2OH���Ҷ�����a����������Ӧ����I������I�ķ���ʽ��֪��aΪCH3CH2COOH��IΪCH3CH2COOCH2CH2OH��
��C��E�������۷�Ӧ��J���Ҷ�����b����������Ӧ����G�����G�ķ���ʽ��֪��bΪCH2=CHCOOH��GΪCH2=CHCOOCH2CH2OH���Ҷ�����a����������Ӧ����I������I�ķ���ʽ��֪��aΪCH3CH2COOH��IΪCH3CH2COOCH2CH2OH��
��1��ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ��CH2=CH2��FΪ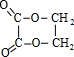 ��GΪCH2=CHCOOCH2CH2OH���Ҷ�����CH2=CHCOOH����������ȡ������Ӧ����G��
��GΪCH2=CHCOOCH2CH2OH���Ҷ�����CH2=CHCOOH����������ȡ������Ӧ����G��
�ʴ�Ϊ��CH2=CH2��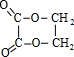 ��������ȡ������Ӧ��
��������ȡ������Ӧ��
��2����C+E��J���Ҷ������Ҷ�������������Ӧ��Ӧ���ɸ߾���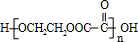 ����Ӧ����ʽΪ��nCH2OHCH2CH2OH+nHOOCCOOH$\stackrel{����}{��}$
����Ӧ����ʽΪ��nCH2OHCH2CH2OH+nHOOCCOOH$\stackrel{����}{��}$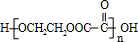 +��2n-1��H2O����G��H��CH2=CHCOOCH2CH2OH��һ�������·����Ӿ۷�Ӧ����Ӧ�Ļ�ѧ����ʽΪ
+��2n-1��H2O����G��H��CH2=CHCOOCH2CH2OH��һ�������·����Ӿ۷�Ӧ����Ӧ�Ļ�ѧ����ʽΪ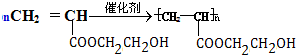 ��
��
�ʴ�Ϊ��nCH2OHCH2CH2OH+nHOOCCOOH$\stackrel{����}{��}$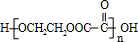 +��2n-1��H2O��
+��2n-1��H2O��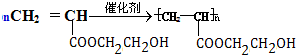 ��
��
��3��IΪCH3CH2COOCH2CH2OH����I��Ϊͬ���칹�壬�ҽṹ�к����Ȼ����ǻ�����CH3CH2CH2CHOHCOOH��CH3CH2CHOHCH2COOH��CH3CHOHCH2CH2COOH��HOCH2CH2CH2CH2COOH����CH3��2COHCH2COOH����CH3��2CHCHOHCOOH��HOCH2CH��CH3��CH2COOH��HOCH2CH2CH��CH3��COOH��CH3CHOHCH��CH3��COOH��CH3CH2C��CH3��OHCOOH��CH3CH2CH��CH2OH��COOH����CH3��2C��CH2OH��COOH����12�֣�
�ʴ�Ϊ��12��CH3CH2CH2CHOHCOOH��CH3CH2CHOHCH2COOH��CH3CHOHCH2CH2COOH��HOCH2CH2CH2CH2COOH����CH3��2COHCH2COOH����CH3��2CHCHOHCOOH��HOCH2CH��CH3��CH2COOH��HOCH2CH2CH��CH3��COOH��CH3CHOHCH��CH3��COOH��CH3CH2C��CH3��OHCOOH��CH3CH2CH��CH2OH��COOH����CH3��2C��CH2OH��COOH��
���� �Կ�ͼ����ʽ�����л�����ƶϣ����ݷ�Ӧ��̼ԭ����Ŀ��ϵȷ������C����2��̼ԭ�����ƶϵĹؼ����ٽ�Ϸ�Ӧ������������ķ���ʽ�����ƶϣ��Ѷ��еȣ���3���ж���������ͬ���칹��Ϊ�״��㣮

| A�� | ��ϩ���Ҵ��� | B�� | ˳����ʯ���ѽ����� | ||
| C�� | �⣨������ | D�� | �壨��ˮɹ�κ����Һ�� |
 ��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������
��֪ij������ľ�������������С��Ԫ���öѻ����ɵģ����ڸû������������������ȷ���ǣ�������| A�� | �û�����Ļ�ѧʽ��Y4Ba4Cu3O12 | B�� | �û�����Ļ�ѧʽ��YBaCu3O6 | ||
| C�� | �û�����Ļ�ѧʽ��Y2BaCu3O6 | D�� | �û�����Ļ�ѧʽ��YBa2Cu3O7 |
| A�� | N2��g��+3H2��g��?2NH3��g����H=-92.4kJ | |
| B�� | 2H2��g��+O2��g���T2H2O��l����H=+571.6KJ•mol-1 | |
| C�� | N2H4��g��+O2��g���TN2��g��+2H2O��g����H=-534.4kJ•mol-1 | |
| D�� | CH4+2O2�TCO2+2H2O��H=-890.3kJ•mol-1 |
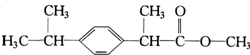 �����л���X��˵���У�������ǣ�������
�����л���X��˵���У�������ǣ�������| A�� | X������ˮ���������л��ܼ� | B�� | 1molX�ܸ�4 mol���������ӳɷ�Ӧ | ||
| C�� | X��ʹ���Ը��������Һ��ɫ | D�� | X��ˮ����ﲻ�ܷ�����ȥ��Ӧ |
| A�� | ͬ����Ԫ�ص�ԭ�Ӱ뾶�Ԣ�A���Ϊ��С | |
| B�� | �����ڱ�������Ԫ�صĵ���ȫ�������� | |
| C�� | ��A����A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ����ʧȥ���� | |
| D�� | ��������Ԫ�ص�ԭ���γɵ�ԭ������ʱ�������������������������� |
HX��aq��?X-��aq��+H+��aq����H��0 K=10-a
X-��aq��+H2O?HX��aq��+OH-��aq����H��0 k=10-b
�����й�Ũ�Ⱦ�Ϊ0.1mol•L-1HX��Һ��NaX��Һ��������ȷ���ǣ�������
| A�� | �ֱ������Һ��ʱ��K��������ҺpH����С | |
| B�� | �����·ֱ�ϡ������Һʱ��K�����䡢��ҺpH������ | |
| C�� | 25��ʱa+b=14 | |
| D�� | 25��ʱ����Һ�������pH=8����Һ�У�c��X-����c��Na+�� |