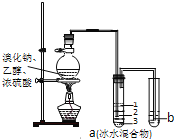
��Ŀ����
����Ŀ�����������ˮ��Һ�еĵ���״�����Խ��ж���������Ʋ⡣
��1��25��ʱ������ĵ���ƽ�ⳣ�������ʾ��
Ka1 | Ka2 | |
HA | 1��10-4 | |
H2B | 1��10-2 | 5��10-6 |
��25��ʱ��0.100mol��L-1��NaA��Һ��H+��OH-��Na+��A-��HA�����ʵ���Ũ���ɴ�С��˳���ǣ�___��pH=8��NaA��Һ����ˮ�������c(OH-)=___mol��L-1��
��25��ʱ��0.100mol��L-1��NaHB��ҺpH___7��������___��
��25��ʱ����0.100mol��L-1��Na2B��Һ�еμ�����0.100mol��L-1��HA��Һ����Ӧ�����ӷ���ʽΪ___��
��2����֪25��ʱ����0.100mol��L-1��H3PO4��Һ�еμ�NaOH��Һ���������������ʵ���������pH�仯�Ĺ�ϵ��ͼ��ʾ��
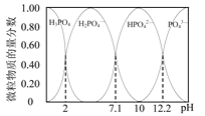
�ٵ���ҺpH��11��14ʱ����������Ӧ�����ӷ���ʽΪ��___��
���𰸡�Na+��A-��OH-��HA��H+ 10-6 �� HB-�ĵ���̶ȴ�����ˮ��̶� HA+B2-=A-+HB- HPO42-+OH-=PO43-+H2O
��������
(1)�ٸ�����Ŀ��Ϣ��֪HAΪ���ᣬ����NaA��Һ�д���A-��ˮ��ʹ��Һ�����ԣ���ˮ�������ģ�ͬʱ��Һ�д���ˮ�ĵ��룬������Һ������Ũ���ɴ�СΪNa+��A-��OH-��HA��H+��pH=8��NaA��Һ��c(H+)=10-8mol��L-1������Һ��c(OH-)=10-6mol��L-1������������ȫ����ˮ���룬������ˮ�������c(OH-)=10-6mol��L-1��
��NaHB��Һ�д���HB-![]() H++B2-�������ƽ�ⳣ��Ϊ5��10-6��ͬʱ����HB-��ˮ�⣬��ˮ��ƽ�ⳣ��Ϊ
H++B2-�������ƽ�ⳣ��Ϊ5��10-6��ͬʱ����HB-��ˮ�⣬��ˮ��ƽ�ⳣ��Ϊ![]() �������ƽ�ⳣ������ˮ��ƽ�ⳣ����������Һ�����ԣ���pH��7��
�������ƽ�ⳣ������ˮ��ƽ�ⳣ����������Һ�����ԣ���pH��7��
�۸�����Ŀ��Ϣ��֪���ԣ�H2B��HA��HB-�����Է�Ӧ�����ӷ���ʽΪHA+B2-=A-+HB-��
(2)��ͼ��֪����ҺpH��11��14ʱ��PO43-Ũ��������HPO42-Ũ���½����������ӷ���ʽΪHPO42-+OH-=PO43-+H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ѱ���Ϊ��21���͵Ľ��������ɳ��ֶ��ֻ��ϼۡ�������+4�۵�Ti��Ϊ�ȶ����ش��������⣺
��1����̬Tiԭ�ӵļ۵����Ų�ͼΪ__��
��2����֪�����ܣ�I2(Ti)=1310kJ��mol-1��I2(K)=3051kJ��mol-1��I2(Ti)<I2(K)����ԭ��Ϊ__��
��3����ij���������ڴ���ϩ���ۺϣ���ṹ��ͼ��ʾ��
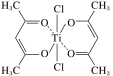
���ѵ���λ��Ϊ__��̼ԭ�ӵ��ӻ�����__��
�ڸ�������д��ڵĻ�ѧ����__(����ĸ)��
a.���ӽ� b.��λ�� c.������ d.���ۼ� e.���
��4������±���γɵĻ������ۡ��е����±���ʾ��
TiCl4 | TiBr4 | TiI4 | |
�۵�/�� | -24.1 | 38.3 | 155 |
�е�/�� | 136.5 | 233.5 | 377 |
����TiCl4��TiBr4��TiI4���۵�ͷе����һ���仯���ɵ�ԭ����__��
��5����֪TiO2��Ũ���ᷴӦ�����������ѣ��������Ѿ�����������Ϊ��״�ۺ���ʽ�����ӣ��ṹ��ͼ��ʾ���������ӻ�ѧʽΪ_�������ӵ����幹��Ϊ__��
![]()
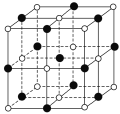
��6����֪TiN����ľ����ṹ��ͼ��ʾ�����þ������ܶ�Ϊ��g��cm-3�������ӵ�����ֵΪNA������Tiԭ����Nԭ�ӵ��������Ϊ___pm��(�ú�����NA�Ĵ���ʽ��ʾ)
����Ŀ����ͭ����Ҫ�ɷ�ΪCu2S�����̿���Ҫ�ɷ�ΪMnO2�����Ƕ��������� SiO2��Fe2O3�����ʡ���ҵ���ۺ����������ֿ����Ʊ�̼���̺�����ͭ�������Ҫ����������ͼ��ʾ ��

��֪���ٲ��ֽ�������������������������� pH��Χ���±���ʾ����ʼ������pH ����������Ũ��Ϊ 0. l mol/L ���㣩
��ʼ������ pH | ������ȫ��pH | |
Fe3+ | 1.1 | 3.2 |
Mn2+ | 8.3 | 9.8 |
Cu2+ | 4.4 | 6.4 |
��100.8��6.3
��1�����ʱ�����д�ʩ�ܹ���߽�ȡ���ʵĴ�ʩ��__________________ ��
A.����ʯ���� B.�ʵ��ӳ����ʱ�� C.�ʵ������¶�
��2�����ʱ�� MnO2 ��Cu2S��Ӧ�����ӷ���ʽ�� __________________��
��3������Һ����pH = 4 ��Ŀ����_____________________ ������Һ��ͭ����Ũ������ܳ���_______________ molL-1������һλС������
��4������ MnCO3 ���������ӷ���ʽ��_______________________��
��5���������п�ѭ��ʹ�õ������� ___________________��д��ѧʽ����
��6����������Ҫ�������HNO3��Һ������ʹ Cu2(OH)2CO3��ȫ�ܽ��⣬��һ�����������ᾧʱ_______________________��
��7���Ƶõ�Cu(NO3)2 ������Ҫ��һ�������� ��ʵ�����������______________ ��
����Ŀ��̼���仯����㷺��������Ȼ�硣���ſƼ��Ľ��������û�ѧ��Ӧԭ������̼���ʽ��к���ת�����ѳ�Ϊ��Դ���á���������������ע����Ľ��㡣��CO2�����ҵ�����ŷŵ���Ҫ���������壬����CO2�������ֵ��ѧƷ��Ŀǰ���о��ȵ㡣
��1��������CH4��CO2���������������������ˮú������֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H =![]() 890.3 kJ��mol��1
890.3 kJ��mol��1
CO(g)��H2O (g)��CO2(g)��H2 (g) ��H =+2.8 kJ��mol��1
2CO(g)��O2(g)��2CO2(g) ��H =![]() 566.0 kJ��mol��1
566.0 kJ��mol��1
��ӦCO2(g)��CH4(g) ![]() 2CO(g)��2H2(g) �ġ�H =____kJ��mol��1
2CO(g)��2H2(g) �ġ�H =____kJ��mol��1
��250��ʱ�������Ͻ�Ϊ��������4 L����������ͨ��6 mol CO2��6 mol CH4���������·�Ӧ��CO2 (g)��CH4(g) ![]() 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
���� | CH4 | CO2 | CO | H2 |
������� | 0.1 | 0.1 | 0.4 | 0.4 |
���¶��¸÷�Ӧ��ƽ�ⳣ��K=_______��
��2������CO2��������Ժϳ��Ҵ�����Ӧԭ��Ϊ��2CO2(g)+6H2(g) ![]() C2H5OH(g)+3H2O(g) H<0,��mΪ��ʼʱ��Ͷ�ϱȣ���m= n(H2)/ n(CO2)��
C2H5OH(g)+3H2O(g) H<0,��mΪ��ʼʱ��Ͷ�ϱȣ���m= n(H2)/ n(CO2)��

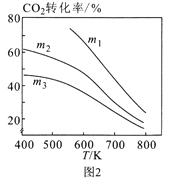
��ͼ1��Ͷ�ϱ���ͬ���¶ȴӸߵ��͵�˳��Ϊ____��
��ͼ2��m1��m2��m3�Ӵ�С��˳��Ϊ____��
��3�����µ�⼼���ܸ�Чʵ�����з�Ӧ��CO2+H2O ![]() CO+H2+O2����ɽ��ͷŵ�CO2ת��Ϊ���й�ҵ���ü�ֵ�IJ�Ʒ������ԭ��ʾ��ͼ���£�
CO+H2+O2����ɽ��ͷŵ�CO2ת��Ϊ���й�ҵ���ü�ֵ�IJ�Ʒ������ԭ��ʾ��ͼ���£�
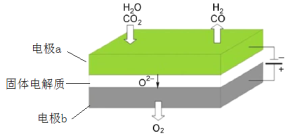
�缫a�ĵ缫��Ӧʽ____
��4����ҵ���������е�CO2������֮һ�ǰ�ˮ��Һ���ռ�������������ȴ��15.5�桫26.5����ð�ˮ���չ�����CO2����֪��NH3��H2O��Kb=1.7��10��5��H2CO3��Ka1=4.3��10��7��Ka2=5.6��10��11�����պ�������Һ��pH____7��������������=��������������
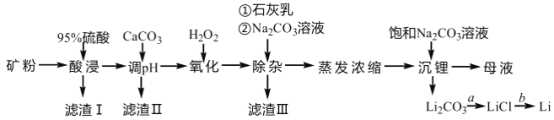
����Ŀ��﮻�ʯ���ҹ���Ҫ�����Դ֮һ������Ҫ�ɷ�Ϊ Li2O��SiO2��Al2O3 �Լ��������� Na+��Fe2+��Fe3+��Ca2+��Mg2+�Ƚ������ӡ���ҵ����﮻�ʯ�Ʊ�����﮵Ĺ����������£�
��֪���ٲ���������������������ʽ����ʱ��Һ�� pH ���±���
������ | Al(OH)3 | Fe(OH)2 | Fe(OH)3 | Ca(OH)2 | Mg(OH)2 |
��ȫ������pH | 5.2 | 9.6 | 3.2 | 13.1 | 10.9 |
�ڳ����£�Ksp(Li2CO3)= 2.0��10-3��Li2CO3 ��ˮ���ܽ�������¶����߶���С��
����ˮ����ʱ��LiCl ���ȿɷ���ˮ�⡣
�ش��������⣺
(1)Ϊ�������������ʣ����������в�ȡ�Ĵ�ʩ��_____��
(2)���� I ����Ҫ�ɷ���_____������ II ����Ҫ�ɷ��� Fe(OH)3��Al(OH)3���������� ����������������Ҫ��ȥ��������_________��
(3) ������������з�����Ӧ�����ӷ���ʽ��_____������ˮϴ�� Li2CO3 ���壬��������ˮϴ�ӣ���ԭ����_____��
(4)��Ƽ�ʵ�鷽��ʵ���ɹ��� a ��ȡ���� LiCl��_____��
(5)��ҵ��ʵ�ֹ��� b ���õķ�����_____��
(6)Li �������Ʊ���Ҫ��ԭ��������ﮣ�LiAlH4�������л��ϳ��У���ԭ���Ļ�ԭ������������Ч������ʾ���京��Ϊ 1�˻�ԭ���൱�ڶ��ٿ� H2 �Ļ�ԭ������LiAlH4 ���� ��Ч����Ϊ_____������ 2 λС������
����Ŀ�����Ȼ�����SnCl4���������л�������������������������ijѧϰС�����ø�������������ڵ����Ʊ� SnCl4 ���ⶨ��Ʒ�Ĵ��ȡ�

��֪��i��SnCl4 �ڿ����м���ˮ��� SnO2��xH2O��ii���й����ʵ������������±���ʾ��
���� | ��ɫ | �۵�/�� | �е�/�� |
Sn | ����ɫ | 232 | 2260 |
SnCl2 | ��ɫ | 247 | 652 |
SnCl4 | ��ɫ | -33 | 114 |
�ش��������⣺
(1)װ�� E ������������_____��װ�� C ��ʢ�ŵ��Լ���_____��
(2)װ�� G ��������_____��
(3)ʵ�鿪ʼʱ����ȷ�IJ���˳��Ϊ_____��
�ٵ�ȼװ�� A ���ƾ��� �ڵ�ȼװ�� D ���ƾ��� �۴�Һ©������
(4)�õ��IJ�Ʒ�Ի�ɫ����������_____�����������м��뵥��___________�����������ƣ�����ȥ��Ϊ�˽�һ�������ᴿ��Ʒ����ȡ�IJ���������_____��
(5)��Ʒ�к����� SnCl2���ⶨ��Ʒ���ȵķ�����ȡ 0.400 g ��Ʒ��������ϡ�����У����������Һ��ָʾ������ 0.0100 mo/L ����ر���Һ�ζ����յ㣬���ı�Һ 8.00 mL ��
�ٵζ�ԭ�����£���� i ��Ӧ�����ӷ���ʽ��
i��_____Sn2++_____IO3��+_____H+= ________Sn4++_____I��+_____
ii��IO3��+5 I2��+6H+ = 3I2 + 3HO
�ڵζ��յ��������_____��
�۲�Ʒ�Ĵ���Ϊ_____%������һλС������
����Ŀ��Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3������������MgO��SiO2�����ʣ����Ʊ��������������£�
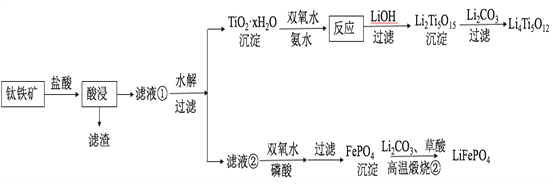
�ش��������⣺
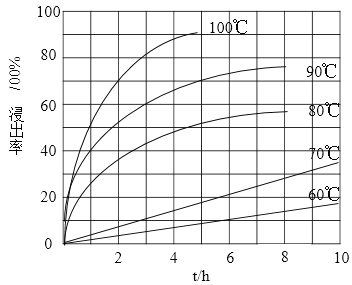
��1���������ʵ���У����Ľ����ʽ������ͼ��ʾ����ͼ��֪�������ľ�����Ϊ70%ʱ�������õ�ʵ������Ϊ___________________��
��2���������������Ҫ��TiOCl42����ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ__________________��
��3��TiO2��xH2O������˫��ˮ����ˮ��Ӧ40 min����ʵ�������±���ʾ��
�¶�/�� | 30 | 35 | 40 | 45 | 50 |
TiO2��xH2Oת����% | 92 | 95 | 97 | 93 | 88 |
����40��ʱTiO2��xH2Oת������ߵ�ԭ��__________________��
��4��Li2Ti5O15��Ti�Ļ��ϼ�Ϊ+4�����й���������ĿΪ__________________��
��5��������Һ�ڡ���c(Mg2+)=0.02 mol/L������˫��ˮ�����ᣨ����Һ�������1������ʹFe3+ǡ�ó�����ȫ����Һ��c(Fe3+)=1��10-5 mol/L����ʱ�Ƿ���Mg3(PO4)2�������ɣ�___________����ʽ���㣩��
FePO4��Mg3(PO4)2��Ksp�ֱ�Ϊ1.3��10-22��1.0��10-24��
��6��д�����������բڡ�����FePO4�Ʊ�LiFePO4�Ļ�ѧ����ʽ______��