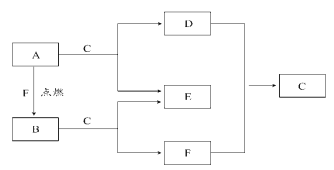
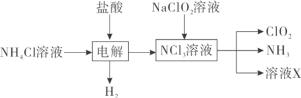
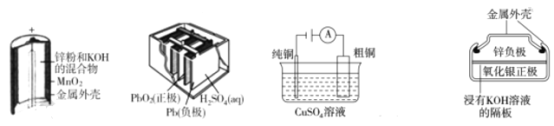
��Ŀ����
����Ŀ����1���ҹ��Ǽ��⼼���Ƚ��Ĺ��ң��챦ʯ��Al2O3�����������ڲ�������IJ��ϡ��������ӷ���ʽ��֤������һ�����������____________��___________��
��2��ȡ������������ijþ���Ͻ�ֱ����������ϡ���������������Һ�У������ı�״����H2����ֱ�Ϊ33.6L��22.4L��úϽ���þ���������ʵ���֮��Ϊ___________��
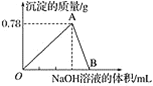
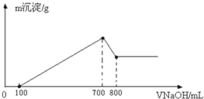
��3����һ��������þ���Ͻ��ܽ���500mL�����У���Ӧ�����Һ����μ���2mol/LNaOH��Һ�������������������Һ����Ĺ�ϵ��ͼ��ʾ������������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����ı仯���Բ��ƣ�_______________�������������������ֵ_____________g��
���𰸡�Al2O3+6H+=2Al3++3H2O Al2O3+2OH��=2AlO2��+H2O 3:4 2.8mol/L 33
��������
��1����������������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�����ų�������
��2������Mg+2HCl=MgCl2+ H2����2Al+6HCl=2AlCl3+3 H2����2Al+2NaOH+2H2O=2 NaAlO2+3 H2�����м��㣻
��3����ͼ���֪��������NaOH700mLʱ�����������ֵ����ʱ��Һ����Һ����ΪNaCl�����������ӡ��������غ��֪n��HCl��=n��NaCl��=n��NaOH�����ٸ���c=![]()
��������Ũ�ȣ�����NaOH800mLʱ������Al��OH��3+NaOH=NaAlO2+2H2O������������ȫ�ܽ⣬����NaOH100mL����ʱn��NaOH��=0.1L��2mol/L=0.2mol���Դ˿ɼ���Al��OH��3�����ʵ���������NaOH��Һ100mL��700mLʱ������Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3������ϸý����ĵ�NaOH����n[Mg��OH��2]���ٸ���m=nM���㣮
��1����������������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�����ӷ���ʽ�ֱ�Ϊ��Al2O3+6H+�T2Al3++3H2O��Al2O3+2OH-�T2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+6H+�T2Al3++3H2O��Al2O3+2OH-�T2AlO2-+H2O��
��2����þ�����ʵ�����xmol���������ʵ�����2ymol��þ���Ͻ���뵽����������Һʱ��ֻ����2Al+2NaOH+2H2O=2 NaAlO2+3 H2�������ݷ�Ӧ�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ���֪�������������ʵ�����3ymol��������֪3y=1mol���ɵ��������ʵ�����![]() ͬ���õ����ĺϽ������ᷴӦʱ�������ᷴӦ�ų�����������ڱ�״������Ӧ����22.4L��˵��û�����ᷴӦ���������������11.2L������£�����Mg+2HCl=MgCl2+ H2������֪þ�����ʵ�����0.5mol��þ���������ʵ���֮��Ϊ0.5mol��
ͬ���õ����ĺϽ������ᷴӦʱ�������ᷴӦ�ų�����������ڱ�״������Ӧ����22.4L��˵��û�����ᷴӦ���������������11.2L������£�����Mg+2HCl=MgCl2+ H2������֪þ�����ʵ�����0.5mol��þ���������ʵ���֮��Ϊ0.5mol��![]() =3:4��
=3:4��
�ʴ�Ϊ��3:4��
(3)��ͼ���֪��������NaOH700mLʱ,���������ֵ,��ʱ��Һ����Һ����ΪNaCl�����������ӡ��������غ��֪��n(HCl)=n(NaCl)=n(NaOH)=0.7L��2moL/L=1.4mol��
����������ʵ���Ũ��=1.4mol0.5L=2.8mol/L��
����������ʵ���Ũ��Ϊ2.8mol/L��
����NaOH800mLʱ������Al(OH)3+NaOH=NaAlO2+2H2O������������ȫ�ܽ⣬����NaOH100mL����ʱn(NaOH)=0.1L��2mol/L=0.2mol����֪Al(OH)3�����ʵ���=0.2mol������NaOH��Һ100mL700mLʱ������Mg2++2OH�TMg(OH)2����Al3++3OH�TAl(OH)3����������������������NaOHΪ0.2mol��3=0.6mol,������������þ����NaOHΪ(0.70.1)L��2mol/L0.6mol=0.6mol,��n[Mg(OH)2]=0.6mol2=0.3mol����������������=0.3mol��58g/mol+0.2mol��78g/mol=33g��
�𣺳������������Ϊ33g.

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�����Ŀ��ʵ�����Ʊ��������Ҫ������������ʾ��

�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gH2O����
�¶� ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | - | - | - | - |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
��ʾ���¶ȸ���35��ʱNH4HCO3��ֽ⣬��ش�
��1�����в�����������ȷ����________��
A���¶ȿ�����30-35������Ϊ�¶�̫��NH4HCO3��ֽ⣬�¶�̫�ͷ�Ӧ����̫��
B������30min��Ŀ����ʹ��Ӧ��ֽ���
C�����˺����Һֻ��NH4Cl��NH4HCO3����
D��ϴȥ�����������ʿ���ѡ������ˮ
��2����Ӧ�¶ȿ�����30��35�棬Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ______________��
��3������ʱ�����˺���Ҫ�õ�NaHCO3�����ԭ����______________��
��4������NaHCO3�����װ��Ϊ________��
A. B.
B. C.
C.
��5��ϴ��NaHCO3����IJ���______________��
��6���ⶨ�����Ʒ��NaHCO3�����ķ�����ȷ��ȡ������ƷWg������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc��mol��L-1����HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ(ָʾCO32-+H+=HCO3����Ӧ���յ�)����HCl��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ���ΪV2mL��д��������Ʒ��NaHCO3���������ļ���ʽ��______________��