��Ŀ����
����Ŀ���ӹ����������仯��������������������ж����˾�����á�
��1���Ŵ��й��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���________��
A��Fe B��FeO C��Fe3O4 D��Fe2O3
��2�����ִ���ҵ�����У������� FeCl3 ��ʴͭ��ԭ������ӡˢ��·�壬д����ԭ���Ļ�ѧ����ʽ_________________________
��3��ʵ���������� FeSO4 ��ҺʱΪ�˷�ֹ FeSO4 ��Һ���ʣ����������м������ۣ���ԭ����_______________(�����ӷ���ʽ��ʾ) ��
��4�������������õ� 100mL 6mol/L FeSO4 ��Һ�������е���һ������ϡ���ᣬ�ش��������⣺
����ƽ�÷�Ӧ�����ӷ���ʽ����Fe2������NO3-����H�� = ��Fe3������NO������H2O___________
��Ҫ����÷�Ӧ�����Һ���Ƿ��� Fe2������ѡ�õ��Լ�Ϊ___________
A. ���� KMnO4 ��Һ B.KSCN ��Һ C. Cl2
�۾����飬������Ӧ�����Һ�в����� Fe2������÷�Ӧ�����в����� NO ���Ϊ����״���£�_____________L��
���𰸡�C 2FeCl3 + Cu = 2FeCl2 + CuCl2 Fe +2Fe3+ = 3Fe2+ 3Fe2����NO3-��4H�� = 3Fe3����NO����2H2O A 4.48
��������
��1��������������һ�־��д��Եĺ�ɫ���壻
��2����������������ͭ����Ϊͭ���ӣ�
��3���������Ӿ��л�ԭ�ԣ�
��4���ٸ��ݵ���غ��ԭ���غ���ƽ����ʽ��
���������Ӿ��л�ԭ�ԣ�
�����ݷ���ʽ���м��㣻
��1��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���������������ѡ��C���ϣ�
��ѡ��C��
��2������ FeCl3 ��ʴͭ��ԭ������ӡˢ��·�壬�����Ӱ�ͭ����������ͭ���ӣ���ѧ����ʽ��2FeCl3 + Cu = 2FeCl2 + CuCl2��
�ʴ�Ϊ��2FeCl3 + Cu = 2FeCl2 + CuCl2��
��3������ FeSO4 ��ҺʱΪ�˷�ֹ FeSO4 ��Һ���ʣ����������м������ۣ���ԭ����Fe +2Fe3+ = 3Fe2+��
�ʴ�Ϊ��Fe +2Fe3+ = 3Fe2+��
��4������Ԫ�صĻ��ϼ���+2������Ϊ+3�ۣ���Ԫ�صĻ��ϼ���+5�۽��͵�+2�ۣ��е��ӣ���ɼ�ԭ���غ��֪���ӷ�ӦΪ3Fe2����NO3-��4H�� = 3Fe3����NO����2H2O��
�ʴ�Ϊ��3Fe2����NO3-��4H�� = 3Fe3����NO����2H2O��
���������Ӿ��л�ԭ�ԣ�����ʹ���Ը��������Һ��ɫ��
��ѡ��A��
�۷�Ӧ�����Һ�в������������ӣ�˵����������ȫ�����뷴Ӧ��n��Fe2����=c��V=0.1L��6mol/L=0.6mol���������ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ��ɵ�n(NO)=![]() n��Fe2����=0.2mol��V(n��Fe2����)=n��Vm=0.2mol��22.4L/mol=4.48L��
n��Fe2����=0.2mol��V(n��Fe2����)=n��Vm=0.2mol��22.4L/mol=4.48L��
�ʴ�Ϊ��4.48

����Ŀ����������ʵ��װ���������Ӧʵ�����
ѡ�� | װ��ͼ | ʵ��Ŀ�� |
A |
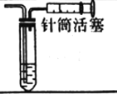
| �ռ������HCl |
B |
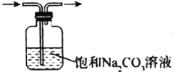
| ��ȥCO2�к��е�����HCl |
C |
| �ռ�H2��NH3��CO2��Cl2��HCl��NO��NO2������ |
D |
| ���װ�õ������� |
A.AB.BC.CD.D