��Ŀ����
����Ŀ��ʵ������Ũ��������1.0mol/L������Һ480mL���ش��������⣺
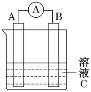
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ______������ĸ��������������Һ�����õ��IJ���������_________�����������ƣ���

��2������ƿ�ϱ�������5���е�_____������ţ�
��ѹǿ ���¶� ������ ��Ũ�� �ݿ̶���
��3�������ƹ����У����в���ʹ������ҺŨ��ƫ�����__________ .
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ��
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���
�۶���ʱ�����ӿ̶���
��ʹ������ƿǰ������ˮϴ����û����
��4������ʵ��������������Һ������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ��������Ϊ��_____mL��������С�����һλ��
���𰸡�A C �ձ��������� �ڢۢ� �� 27.2
��������
����һ�����ʵ���Ũ�ȵ���Һʱ��Ҫ�õ��IJ�������Ϊ�ձ�����������һ����������ƿ����ͷ�ιܡ���Ͳ��������Ũ��Һ����ϡ��Һ�Ĺ����У���������c=��w%/M�����Ũ�����Ũ�ȣ��ٸ�����Һϡ���������ʵ�����δ�����仯��ʹ��cŨVŨ=cϡVϡ��һ��ϵ���м��㡣
��1������һ�����ʵ���Ũ�ȵ���Һ�����п϶�����Ҫ������ƿ�ͷ�Һ©��������һ�����ʵ���Ũ�ȵ���Һ�����õ��IJ����������ձ�������������ӦΪ��A C���ձ� ��������
��2������ƿ��һ��ϸ������ƽ��������������ĥ�ڲ����������б��ߣ���ʾ����ָ�¶���Һ�尼Һ��������ƿ�����ı�������ʱ����Һ���ǡ����ƿ�ϱ�ע�������ȡ�����ƿ�ϱ��У��¶ȡ��������̶��ߡ���˴�Ϊ�ڢۢݣ�
��3�������ƹ����У�
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�У���ʹ��Һ���¶ȹ��߶����ƫ����˶���ʱ�����ˮ��ƫ�٣�ʹ��������ҺŨ��ƫ�ߣ�
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ��������������в�����Һճ��ƿ����ƿ�����������ý�ͷ�ιܼ�����ˮ���̶��ߣ����ˮƫ�࣬��Һ���ƫ��ʹ��������ҺŨ��ƫ�ͣ�
�۶���ʱ�����ӿ̶��ᵼ��������Һ��Һ�����ƫ��Ũ��ƫ�ͣ�
�����ڶ���ʱ����������ƿ�м���ˮ�����ʹ������ƿǰ������ˮϴ����û���ﲻ���ʵ��������Ӱ�졣
��˴�Ӧѡ�٣�
(4)��������Ϊ98�����ܶ�Ϊ1.84g/mL��Ũ��������ʵ���Ũ��Ϊ��c��Ũ���ᣩ��![]() ��18.4moL������Һϡ��ǰ�����ʵ����ʵ������䣬�����Ũ��������ΪV������18.4mo/L��V��1.0mol/L��0.5L��V��0.02717L��������С�����һλʱ��V=27.2mL���ʴ�Ϊ��27.2��
��18.4moL������Һϡ��ǰ�����ʵ����ʵ������䣬�����Ũ��������ΪV������18.4mo/L��V��1.0mol/L��0.5L��V��0.02717L��������С�����һλʱ��V=27.2mL���ʴ�Ϊ��27.2��