��Ŀ����
����Ŀ��A��B��C��D������ͬһ��Ԫ�أ�����֮���ת����ϵ��ͼ��ʾ����Ӧ���������������Ѿ���ȥ�� A ![]() B
B ![]() C
C ![]() D
D
I����A��һ�ֻ�ɫ���嵥�ʣ�BΪ����ij���֮һ���ҿ�ʹƷ����Һ��ɫ���ش��������⣺
��1����Bͨ��KMnO4��Һ������Ϊ��_____������B��____��ѡ����������������ԭ������Ư����������
��2����д��D��Ũ��Һ�뵥��ͭ��Ӧ�Ļ�ѧ����ʽ��_____.
����A�����ʹʪ��ĺ�ɫʯ����ֽ��������ش���������
��1��д��A��B�Ļ�ѧ��Ӧ����ʽ_________
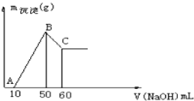
��2��ʵ���ҳ���ͼ��ʾװ����ȡ���ռ�A����,���ڿ��л���ʵ�����ռ�A�����װ��ͼ_____
��3��β������װ����ʹ�õ���©����������_______��
���𰸡���ɫ��Һ��ɫ ��ԭ�� Cu +2H2SO4(Ũ) ![]() CuSO4 + SO2��+2H2O 4NH3 +5O2
CuSO4 + SO2��+2H2O 4NH3 +5O2 ![]() 4NO + 6H2O
4NO + 6H2O  ��ֹ����
��ֹ����
��������
��A�ǻ�ɫ���壬BΪ����ij���֮һ���ҿ�ʹƷ����Һ��ɫ����AΪS��BΪSO2��CΪSO3��DΪH2SO4����A�����ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��BΪNO��CΪNO2�� DΪHNO3���Դ˽��⡣
I����1��BΪ���������л�ԭ�ԣ����������ط���������ԭ��Ӧ��ʹ���������Һ��ɫ����˴�Ϊ����ɫ��Һ��ɫ����ԭ�ԣ�
��2��DΪH2SO4������ǿ�����ԣ��ڼ�����������ͭ����������ԭ��Ӧ�����뵥��ͭ��Ӧ�Ļ�ѧ����ʽ��Cu +2H2SO4(Ũ) ![]() CuSO4 + SO2��+2H2O��
CuSO4 + SO2��+2H2O��
��1��AΪNH3��BΪNO�����A��B�Ļ�ѧ��Ӧ����ʽΪ4NH3 +5O2 ![]() 4NO + 6H2O��
4NO + 6H2O��
��2��ʵ���ҳ���ͼ��ʾװ����ȡ���ռ�NH3����,�ڿ���ӦΪ�����ռ�װ�á�����NH3���ܶȱȿ���ҪС������������ſ����������ռ�����װ��ͼΪ��
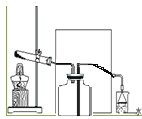
��3�����ڰ�����ˮ�е��ܽ�ȷdz������β������װ����ʹ�õ���©���������Ƿ�������

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�����Ŀ����Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO��NH2��2]����֪��
��2NH3��g����CO2��g����NH2CO2NH4��s����H ����159.47 kJ��mol-1
��NH2CO2NH4��s����CO��NH2��2��s����H2O��g����H ��+116.49 kJ��mol-1
��H2O��l����H2O��g����H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ______________��
��2����֪��
��ѧ�� | Si��Cl | H��H | H��Cl | Si��Si |
����/kJ��mol��1 | 360 | 436 | 431 | 176 |
�ҹ辧����ÿ����ԭ�Ӻ�����4����ԭ���γ�4�����ۼ�����ҵ�����õĸߴ����ͨ������Ӧ����ȡ��SiCl4��g����2H2��g��![]() Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
��3����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�CO2��g��+4H2��g��![]() CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
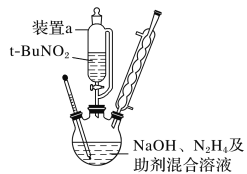
��4���۲���ͼ��ʾ������װ�ã�ͼ1װ����ͭ�缫�ϲ�����������ɫ���ݣ�ͼ2װ����ͭ�缫������������������缫�ϲ�����������ɫ���塣���������������Ʋ���������е�������Ҫ��ѧ����Ϊ
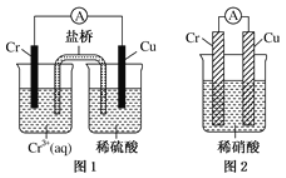
�� ____________________________________��
�� ____________________________________��