题目内容
【题目】现有下列有机物:①CH4,②CH3CH2OH,③ ,④癸烷,⑤CH2=CHCH3,⑥
,④癸烷,⑤CH2=CHCH3,⑥![]() ,⑦
,⑦![]() ,⑧丙烷,⑨
,⑧丙烷,⑨![]() ,⑩C5H12O
,⑩C5H12O
根据上述物质,回答下列问题:
(1)相对分子质量为44的烷烃的结构简式为___。
(2)与③互为同分异构体的是__![]() 填序号
填序号![]() 。
。
(3)具有特殊气味,常作萃取剂的某有机物在FeBr3作催化剂的条件下可与液溴发生取代反应,该反应的化学方程式为__。
(4)有机物②在加热条件下和CuO反应的化学方程式为__。
(5)在120℃,1.01×105Pa条件下,某种气态烃与足量的O2完全反应后,测得反应前后气体的体积没有发生改变,则该烃是__![]() 填序号
填序号![]() 。
。
(6)萘的结构简式是![]() ,其一氯代物有__种。
,其一氯代物有__种。
(7)某摩尔质量为42g/mol的链烃A可使溴水褪色,在一定条件下发生加聚反应的化学方程式___。
(8)分子组成为C5H12O同分异构体中属于醇的有___种。
【答案】CH3CH2CH3 ⑦ 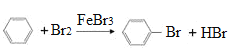 CH3CH2OH+CuO
CH3CH2OH+CuO![]() CH3CHO+Cu+H2O ① 2
CH3CHO+Cu+H2O ① 2 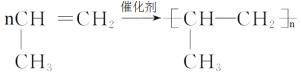 8
8
【解析】
(1)根据烷烃的通式CnH2n+2可知12n+2n+2=44,解得n=3,即为C3H8,结构简式为CH3CH2CH3;
(2)③中有6个碳原子,且属于链烃,与③分子式相同结构不同的是⑦;
(3)具有特殊气味,常作萃取剂的是苯,和液溴在铁作催化剂条件下发生取代反应,其反应方程式为: ;
;
(4)有机物②是乙醇,乙醇被氧化铜氧化成乙醛,本身被还原成Cu,即CH3CH2OH+CuO![]() CH3CHO+Cu+H2O;
CH3CHO+Cu+H2O;
(5)烃燃烧温度高于100℃,则生成的水也气态,反应前后气体的体积没有发生改变,根据燃烧通式![]() ,可知
,可知![]() ,解得m=4,即烃中氢的个数为4,因此为①甲烷;
,解得m=4,即烃中氢的个数为4,因此为①甲烷;
(6)因为萘是轴对称结构 ,上下、左右分别对称,虽然萘一共有8个H,但是根据等效氢理论,1、4、5、8这四种氢原子是等效氢,2、3、6、7这四种氢原子也是等效氢,所以萘一共有2种氢原子,因此只有两种一氯代物;
,上下、左右分别对称,虽然萘一共有8个H,但是根据等效氢理论,1、4、5、8这四种氢原子是等效氢,2、3、6、7这四种氢原子也是等效氢,所以萘一共有2种氢原子,因此只有两种一氯代物;
(7)链烃A摩尔质量为42g/mol的,可得分子式为C3H6,使溴水褪色,含有不饱和键,所以为丙烯,发生加聚反应的化学方程式为 ;
;
(8)分子式C5H12为戊烷,有三种同分异构体:正戊烷、异戊烷和新戊烷,那么C5H12O的同分异构体中属于醇,就是在戊烷上加上一个羟基,按羟基位置不同,有如下8种情况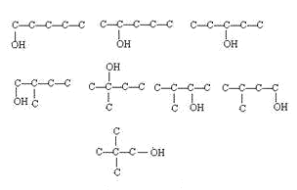 。
。

【题目】某研究性学习小组的同学设计了如图装置制取溴苯和溴乙烷:
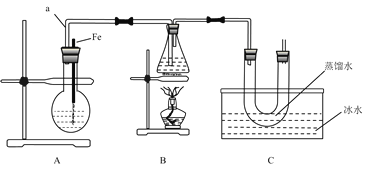
己知:乙醇在加热的条件下可与HBr反应得到溴乙烷(CH3CH2Br),二者某些物理性质如下表所示:
溶解性(本身均可作溶剂) | 沸点(℃) | 密度(g/mL) | |
乙醇 | 与水互溶,易溶于有机溶剂 | 78.5 | 0.8 |
溴乙烷 | 难溶于水,易溶于有机溶剂 | 38.4 | 1.4 |
请回答下列问题:
(1) B中发生反应生成目标产物的化学方程式为_________。
(2)根据实验目的,选择下列合适的实验步骤:①→___________(选填②③④等)。
①组装好装置,___________(填写实验操作名称);
②将A装置中的纯铁丝小心向下插入苯和液溴的混合液中;
③点燃B装置中的酒精灯,用小火缓缓对锥形瓶加热10分钟;
④向烧瓶中加入一定量苯和液溴,向锥形瓶中加入无水乙醇至稍高于进气导管口处,向U形管中加入蒸馏水封住管底,向水槽中加入冰水。
(3)简述实验中用纯铁丝代替铁粉的优点:_____。
(4)冰水的作用是_______。
(5)反应完毕后,U形管内的现象是______________;分离溴乙烷时所需的玻璃仪器有_____。