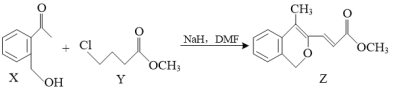��Ŀ����
����Ŀ����11�֣�����������������ӵ�صĹ㷺Ӧ�ã�������ӵ�صĻ��մ���������Ҫ�����������÷�����ӵ���������ϣ���Al��LiCoO2��Ni��Mn��Fe�ȣ������ܡ�����﮵�����ͼ��
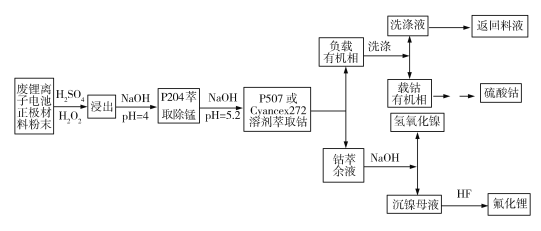
��֪��P204[��(2�һ�����)������]��������ȡ�̣�P507(2�һ��������2�һ���������Cyanex272[��(2��4��4)�������������]��������ȡ�ܡ�����
�ش��������⣺
��1����������ڵ������£��������Ϸ�ĩ��LiCoO2��H2O2��Ӧ������ʹ������ľ����ȼ�����壬��д����Ӧ�Ļ�ѧ����ʽ__________________________________��
��2��һЩ������������������ܽ�ȣ��������ӵı���Ũ�ȱ�ʾ����pH�Ĺ�ϵͼ���£�
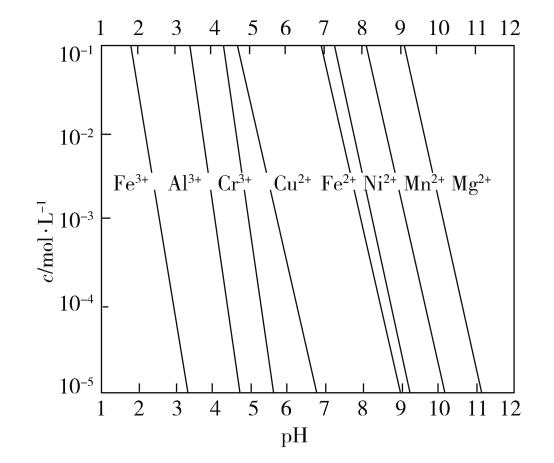
����NaOH��Һ��pH=5�ɳ�ȥͼ�е�________����������ӷ��ţ����ʣ�д����ȥ�������ӵ����ӷ���ʽ________________________��һ�ּ��ɣ���
��3����֪P507��ȡ�������ӵ�ԭ��ΪnHR(Org)+Mn+(aq)![]() MRn(Org)+nH+(aq)����������ȡ������pH���ͣ���ȡЧ���½�����ȡǰ����NaOH����ȡ����������������������ȡ����ȡ�������ӵķ�ӦΪnNaR(Org)+Mn+(aq)
MRn(Org)+nH+(aq)����������ȡ������pH���ͣ���ȡЧ���½�����ȡǰ����NaOH����ȡ����������������������ȡ����ȡ�������ӵķ�ӦΪnNaR(Org)+Mn+(aq)![]() MRn(Org)+nNa+(aq)������ȡ����������������ԭ��Ϊ________________��
MRn(Org)+nNa+(aq)������ȡ����������������ԭ��Ϊ________________��
��4������ˮ��pH=5.2���¶�25�棬�ֱ���P507��Cyanex272����ȡ������ȡ��Ũ�ȶ���ȡ�����ܡ�����Ӱ��ʵ������ͼ��ʾ��
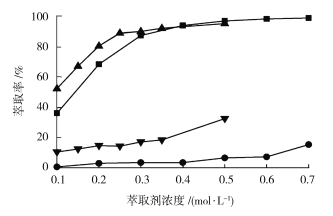
����Co(Cyanex272)����Ni(Cyanex272)������Co(P507)������Ni(P507)
��ͼ��֪���ܡ�������ȡ������ȡ��Ũ�������_________������������������С������������ȡ����___________������P507������Cyanex272�����ķ���Ч���ȽϺã���ѡP507Ϊ��ȡ�����������˵���ȡ��Ũ�ȴ�ԼΪ__________mol��L1����ѡCyanex272��ȡ�����������˵���ȡ��Ũ�ȴ�ԼΪ___________mol��L1��
��5�������£���NaOH��Һ����������Һ��pH=12������һ��ʱ����ã����ķ���õ�����ɫ�����������壬�������ʿɴ�99.62%����֪Ksp[Ni(OH)2]=5.25��1016�������ĸҺ��Ni2+��Ũ��Ϊ2.1��1011 mol��L1ʱ��pH=______(lg5=0.7)��
���𰸡�2LiCoO2+3H2SO4+H2O2![]() Li2SO4+O2��+2CoSO4+4H2O Fe3+��Al3+��1�֣���ѡ�ʹ�ѡû�֣� Fe3+ +3OH
Li2SO4+O2��+2CoSO4+4H2O Fe3+��Al3+��1�֣���ѡ�ʹ�ѡû�֣� Fe3+ +3OH![]() Fe(OH)3��Al3+ +3OH
Fe(OH)3��Al3+ +3OH![]() Al(OH)3���������ɣ� ά����ȡ������pH�㶨 ���� Cyanex272 0.25 0.4 11.7
Al(OH)3���������ɣ� ά����ȡ������pH�㶨 ���� Cyanex272 0.25 0.4 11.7
��������
��1��LiCoO2�������H2O2��Ӧ������ʹ������ľ����ȼ�����壬˵��LiCoO2�����������°�H2O2������O2��+3��Co����Ϊ+2�۵�Co2+������ʽΪ2LiCoO2+3H2SO4+H2O2![]() Li2SO4+O2��+
Li2SO4+O2��+
2CoSO4+4H2O��
��2����ͼ��֪������NaOH��Һ��pH=5ʱ��Fe3+��Al3+������ȫ������ȥ��Fe3+�����ӷ���ʽΪFe3++3OH![]() Fe(OH)3��ȥ��Al3+�����ӷ���ʽΪAl3++3OH
Fe(OH)3��ȥ��Al3+�����ӷ���ʽΪAl3++3OH![]() Al(OH)3��
Al(OH)3��
��3��nHR(Org)+Mn+(aq)![]() MRn(Org)+ nH+(aq)����ʹ��Һ������ǿ����ȡЧ���½�������NaOH�����������������ӷ�Ӧ��ΪnNaR(Org)+Mn+(aq)
MRn(Org)+ nH+(aq)����ʹ��Һ������ǿ����ȡЧ���½�������NaOH�����������������ӷ�Ӧ��ΪnNaR(Org)+Mn+(aq)![]() MRn(Org)+nNa+(aq)����Ӧǰ��pH�������䣬���������֪����ȡЧ�ʲ��ή�͡�����ȡǰ����NaOH����ȡ����������������Ŀ����ά����ȡ������pH�㶨��
MRn(Org)+nNa+(aq)����Ӧǰ��pH�������䣬���������֪����ȡЧ�ʲ��ή�͡�����ȡǰ����NaOH����ȡ����������������Ŀ����ά����ȡ������pH�㶨��
��4����ͼ��֪���ܡ�������ȡ������ȡ��Ũ����������������ƣ���ȡʱ��Cyanex272������P507�����ܡ�����ȡ�ʵIJ�ֵ��Cyanex272����Ч���á�ѡP507Ϊ��ȡ����Ũ����0.25 mol��L1�Ժ�仯��������0.25 mol��L1��ã�ѡCyanex272��ȡ����Ũ����0.40 mol��L1�Ժ�仯��������0.4 mol��L1��á�
��5��Ksp[Ni(OH)2]=c(Ni2+)��c2(OH)=5.25��1016��c2(OH)=![]() =2.5��105��c(OH)=5��103��pOH=3lg5=2.3��pH=14pOH=142.3=11.7��
=2.5��105��c(OH)=5��103��pOH=3lg5=2.3��pH=14pOH=142.3=11.7��

 ȫ�ܲ��һ���þ�ϵ�д�
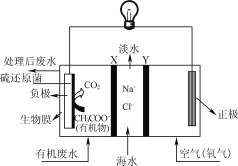
ȫ�ܲ��һ���þ�ϵ�д�����Ŀ����Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
��1����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO��NH2��2]����֪��
��2NH3��g����CO2��g����NH2CO2NH4��s����H ����159.47 kJ��mol-1
��NH2CO2NH4��s����CO��NH2��2��s����H2O��g����H ��+116.49 kJ��mol-1
��H2O��l����H2O��g����H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ______________��
��2����֪��
��ѧ�� | Si��Cl | H��H | H��Cl | Si��Si |
����/kJ��mol��1 | 360 | 436 | 431 | 176 |
�ҹ辧����ÿ����ԭ�Ӻ�����4����ԭ���γ�4�����ۼ�����ҵ�����õĸߴ����ͨ������Ӧ����ȡ��SiCl4��g����2H2��g��![]() Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
Si��s����4HCl��g�����÷�Ӧ����H��___ kJ��mol��1��
��3����һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�CO2��g��+4H2��g��![]() CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
CH4��g��+2H2O��g�� ��H��0��һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
��4���۲���ͼ��ʾ������װ�ã�ͼ1װ����ͭ�缫�ϲ�����������ɫ���ݣ�ͼ2װ����ͭ�缫������������������缫�ϲ�����������ɫ���塣���������������Ʋ���������е�������Ҫ��ѧ����Ϊ
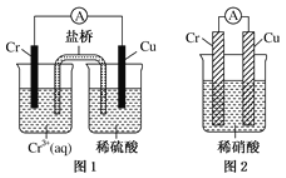
�� ____________________________________��
�� ____________________________________��
����Ŀ��ij�о���ѧϰС���ͬѧ�������ͼװ����ȡ�屽�������飺
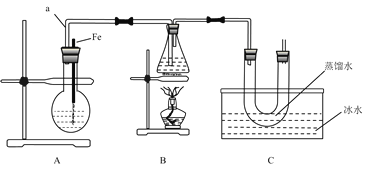
��֪���Ҵ��ڼ��ȵ������¿���HBr��Ӧ�õ������飨CH3CH2Br��������ijЩ�����������±���ʾ��
�ܽ��ԣ������������ܼ��� | �е㣨�棩 | �ܶȣ�g/mL�� | |
�Ҵ� | ��ˮ���ܣ��������л��ܼ� | 78.5 | 0.8 |
������ | ������ˮ���������л��ܼ� | 38.4 | 1.4 |
��ش��������⣺
��1�� B�з�����Ӧ����Ŀ�����Ļ�ѧ����ʽΪ_________��
��2������ʵ��Ŀ�ģ�ѡ�����к��ʵ�ʵ�鲽�裺�١�___________��ѡ��ڢۢܵȣ���
����װ��װ�ã�___________����дʵ��������ƣ���
�ڽ�Aװ���еĴ���˿С�����²��뱽��Һ��Ļ��Һ�У�
�۵�ȼBװ���еľƾ��ƣ���С������ƿ����10���ӣ�
������ƿ�м���һ��������Һ�壬����ƿ�м�����ˮ�Ҵ����Ը��ڽ������ܿڴ�����U�ι��м�������ˮ��ס�ܵף���ˮ���м����ˮ��
��3������ʵ�����ô���˿�������۵��ŵ㣺_____��
��4����ˮ��������_______��
��5����Ӧ��Ϻ�U�ι��ڵ�������______________������������ʱ����IJ���������_____��