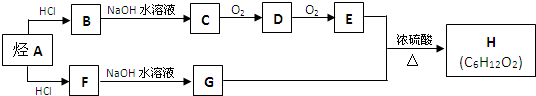
��Ŀ����
13������Fe3++Ag?Fe2++Ag+������Fe3+������Һ����ʴҺ���Թ��е�����ϴȥ����1��FeCl3��Һ�����ԣ�ԭ����Fe3++3H2O?Fe��OH��3+3H+�������ӷ���ʽ��ʾ������
��2������FeCl3��Һϴ��������������ȷ����a������ţ���
a��c ��Fe3+����С b��c��Cl-������ c����Ԫ��������С
��3��Fe��N03��3��Һϴ��ʱ����ͬѧ��ΪNO3һҲ�ܽ�������������Ϊ����ͨ������Fe��N03��3��Һϴ����NO3һ�Ļ�ԭ�������ж�NO3һ�Ƿ��ܽ�������������ͬѧ��Ϊ�˷��������У�����С������У���������ͬѧ������������������NO3-Ҳ������Fe2+����������ԭ��Ӧ
��4����ͬѧ����ϴ����Һ��Fe3+��Fe2+��Ag+��NO3һ���л������Ϳ�ʴҺ���������ͼ·�ߣ�

�ٹ���I�з�Ӧ�����ӷ���ʽ��2Fe3++Fe=3Fe2+��Fe+2Ag+=Fe2++2Ag��
�ڹ���II�м�����Լ������ǣ�ϡ�����ϡ����
��5���������������Һ����ʱ��������ǿ��ը�Ե����ʣ����Բ������森��������Һ�л������ķ����ǣ���������Һ�м���������ᣬ���ˣ������AgCl�м����ǰ���NH2OH������ַ�Ӧ��ɵ������ᰱ������ΪN2��
������AgCI�����Ļ�ѧ����ʽ��Ag��NH3��2OH+3HCl=AgCl��+2NH4Cl+H2O��
�����÷�Ӧ������3.3g�ǰ��������Ͽɵ���������Ϊ10.8g��
���� ��1��������ˮ���������������������ӣ���Һ�����ԣ�
��2�����ݷ�ӦFe3++Ag?Fe2++Ag+�������ж������Ӳ��䣬��Ԫ���������䣻
��3�����������������Һ�о��������ԣ��������������������ӣ�
��4����ϴ����Һ��Fe3+��Fe2+��Ag+��NO3-���л������Ϳ�ʴҺ��Ҫ�ȼ����������ԭFe3+��Ag+��
���Լ������ܽ����������
��5����������Һ�м���������������Ȼ�����
�������AgCl�м����ǰ���NH2OH������ַ�Ӧ��ɵ������ǰ�������ΪN2����ӦΪ��2AgCl+2NH2OH=N2��+2Ag+2H2O+2HCl�����ݷ�Ӧ���㣮
��� �⣺��1��FeCl3��Һ����������Ϊ������ˮ���������������������ӣ���Ӧ�����ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
��2��FeCl3��Һϴ����������ӦΪ��Fe3++Ag?Fe2++Ag+��
a��c��Fe3+����С����a���ϣ�������
b�� c��Cl-���ı䣬��b�����ϣ�������
c����Ԫ�ش�����ʽ��ͬ�����������С����c�����ϣ�
�ʴ�Ϊ��a��
��3��ͨ������Fe��NO3��3��Һϴ����NO3-�Ļ�ԭ�������ж�NO3-�Ƿ��ܽ�����������Ҫ������Һ�У���������Ҳ�ᷴӦ����÷��������У�
�ʴ�Ϊ�������У�����������NO3-Ҳ������Fe2+����������ԭ��Ӧ��
��4����ϴ����Һ��Fe3+��Fe2+��Ag+��NO3-���л������Ϳ�ʴҺ��Ҫ�ȼ����������ԭFe3+��Ag+����Ӧ�����ӷ���ʽΪ��2Fe3++Fe=3Fe2+��Fe+2Ag+=Fe2++2Ag��
�ʴ�Ϊ��2Fe3++Fe=3Fe2+��Fe+2Ag+=Fe2++2Ag��
�ڹ��̢��м�����Լ����ܽ����������������ϡ�����ϡ���
�ʴ�Ϊ��ϡ�����ϡ���
��5����������Һ�м���������������Ȼ�����ͬʱ�����Ȼ�狀�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ag��NH3��2OH+3HCl=AgCl��+2NH4Cl+H2O��
�ʴ�Ϊ��Ag��NH3��2OH+3HCl=AgCl��+2NH4Cl+H2O��
�������AgCl�м����ǰ���NH2OH������ַ�Ӧ��ɵ������ǰ�������ΪN2����ӦΪ��2AgCl+2NH2OH=N2��+2Ag+2H2O+2HCl����Ӧ������3.3g�ǰ����ʵ���=$\frac{3.3g}{33g/mol}$=0.1mol�����������ʵ���Ϊ0.1mol������Ϊ10.8g��
�ʴ�Ϊ��10.8��
���� ���⿼�����ʵ�����ʵ�飬Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬��Ŀע�ػ������õ���ȡ������������жϣ��Լ�ѡ���Ŀ�ģ����ӷ���ʽ��ѧ����ʽ��д�ķ������������������ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | ʳ��ˮ����ˮ | B�� | ��ɳ��ˮ | C�� | ������Ȼ�̼ | D�� | ˮ�����Ȼ�̼ |
| A�� | ���� | B�� | ���� | C�� | CO2 | D�� | ��ˮ |
| A�� | ��ˮ�м��백ˮ��ƽ�������ƶ���c��OH-������ | |
| B�� | ��ˮ�м����������ƣ�ƽ�������ƶ���c��H+������ | |
| C�� | ��ˮ�м������������������ƣ�c��H+������KW���� | |
| D�� | ��ˮ���ȣ�KW����pH���� |
| A�� | $\frac{a+b-2c}{4}$kJ | B�� | $\frac{a+2b-4c}{8}$kJ | C�� | $\frac{b-a-2c}{4}$kJ | D�� | $\frac{2b-a-4c}{8}$kJ |
| A�� | ȡ��NaOH����Һǰ������ˮϴ����ƿ | |
| B�� | ��ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ���� | |
| C�� | ҡ����ƿʱ����������Һ����ƿ�� | |
| D�� | �ζ�ǰ�ζ����������ݣ��ζ�����ʧ |
| X | Y | ||
| Z | W |
| A�� | X��Y��Z��W����Ԫ�ؾ�Ϊ�ǽ���Ԫ�� | |
| B�� | Y��W������⻯���У�Y�ķе�� | |
| C�� | X��Y��Z��W����������Ϊ���ۻ����� | |
| D�� | WԪ�صļ����Ӱ뾶С��ZԪ�صļ����Ӱ뾶 |

 CH3CH2COOCH��CH3��2+H2O��
CH3CH2COOCH��CH3��2+H2O��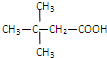 �����ýṹ��ʽ��ʾ��
�����ýṹ��ʽ��ʾ��