��Ŀ����
17�������������ڸ��������£����ӹ����жϼ���Ӧ�����ӷ���ʽ����ȷ���ǣ�������| ѡ�� | �� �� | ������ | ���ӹ����жϼ����ӷ���ʽ |
| A | �����£���ˮ�������C��H+��Ϊ1��10-12 mol•L-1 | K+��Cl-��S2-SO32- | һ���ܴ������� |
| B | �μӰ�ˮ | Na+��Fe3+��Cl- | ���ܴ������� Fe3++3OH-�TFe��OH��3�� |
| C | p=1����Һ | Fe3+��I- Cl- | ���ܴ������� 2Fe3++2I-�T2Fe2++I2 |
| D | ͨ������SO2���� | K+��Na+��ClO- | ���ܴ������� 2ClO-+SO2+H2O�T2HClO+SO32- |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A����ˮ�������H+Ũ��Ϊ1��10-12mol•L-1��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ������ӡ�������������������ӷ�Ӧ��
B��һˮ�ϰ�Ϊ������ʣ�Ӧ������ѧʽ��
C��pH=1����Һ�����ԣ�������������ӷ���������ԭ��Ӧ��
D��SO2���л�ԭ�ԣ���ClO-����������ԭ��Ӧ��
��� �⣺A����ˮ�������H+Ũ��Ϊ1��10-12mol•L-1��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�S2-��SO32-��������Һ�е������ӷ�Ӧ����������Һ�в��ܴ������棬��A����
B��Na+��Fe3+��Cl-����֮�䲻������Ӧ���μӰ�ˮ�������ӷ�Ӧ���������������������ܴ������棬����һˮ�ϰ�Ϊ������ʣ���ȷ�����ӷ���ʽΪ��Fe3++3NH3•H2O�TFe��OH��3��+3NH4+����B����
C��pH=1����Һ�����ԣ�Fe3+��I-����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��2Fe3++2I-�T2Fe2++I2���жϺ����ӷ���ʽ����������C��ȷ��
D��SO2���л�ԭ�ԣ���ClO-����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��ClO-+SO2+H2O�T2H++Cl-+SO42-���������ӷ���ʽ����D����
��ѡC
���� ���⿼�����ӹ���������жϣ�Ϊ�е��Ѷȵ����⣬ע����ȷ���Ӳ��ܴ��������һ��������磺�ܷ������ֽⷴӦ������֮�䣻�ܷ���������ԭ��Ӧ������֮��ȣ�������ע��Ҫ�����ӹ����жϼ���Ӧ�����ӷ���ʽ����ȷ����Ϊ�״��㣮

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�| A�� | �����ڹ��ۼ�Խǿ������Խ�ȶ������ۡ��е�ҲԽ�� | |
| B�� | ���ۻ���������ˮ�������ڹ��ۼ�һ�����ƻ� | |
| C�� | ���Ӽ����γ�һ���е��ӵĵ���ʧ | |
| D�� | ���Ӿ�����һ�����ڷ��Ӽ�����������һ�����ڹ��ۼ� |
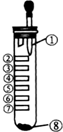 �������ʵ���ʵ��װ����ͼ��ʾ������Ũ���ᣬ���Ǹ�����أ�����������ֽ�������ε���Ʒ ����Һ��ʯ����Һ������KI��Һ��Na2S ��Һ��KBr ��Һ����KSCN ��FeCl2������Һ��ʵ��ʱ������Ũ���ᣬ����˵��������ǣ�������
�������ʵ���ʵ��װ����ͼ��ʾ������Ũ���ᣬ���Ǹ�����أ�����������ֽ�������ε���Ʒ ����Һ��ʯ����Һ������KI��Һ��Na2S ��Һ��KBr ��Һ����KSCN ��FeCl2������Һ��ʵ��ʱ������Ũ���ᣬ����˵��������ǣ�������| A�� | �������������ӷ���ʽ��16H++10Cl-+2MnO${\;}_{4}^{-}$=2Mn2++5Cl2��+8H2O | |
| B�� | �ߴ���Ѫ��ɫ������Ϊ2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3 | |
| C�� | ����ɫ���۴��ȱ�����ɫ���ݴ����ֵ���ɫ���� | |
| D�� | �ܴ�����������Ⱥ죬��˵�������ԣ�Cl2��Br2��I2 |
��������
| A�� | һ�������£��÷�Ӧ���κ��¶��¾����Է����� | |
| B�� | ��ӦCO��g��+Cl2��g��?COCl2��g����H=+Q kJ•mol-1 ��ѧƽ�ⳣ��ΪK | |
| C�� | ��Ӧ2COCl2��g��?2CO��g��+2Cl2��g�� ��ѧƽ�ⳣ��Ϊ2K | |
| D�� | ��1mol COCl2 ��g������һ�ܱ������г�ַ�Ӧ��ų�QkJ������ |
| A�� | �л���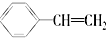 ���ڷ��������������ֹ����� ���ڷ��������������ֹ����� | |
| B�� | ��ϵͳ�������������CH3��2CHCH��CH3��CH2CH��CH3�� CH��CH3��2������Ϊ2��3��5��6-�ļ����� | |
| C�� | 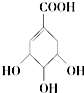 �����ڷ��������������� �����ڷ��������������� | |
| D�� | 2��3��ϩ����������ԭ����ѡ�������� |
| A�� | 0.1 mol•L-1 | B�� | 1 mol•L-1 | C�� | 3 mol•L-1 | D�� | 1.5 mol•L-1 |

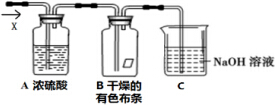 ijͬѧӦ����ͼ��ʾװ���о����ʵ����ʣ���������X����Ҫ�ɷ�������������������������ˮ��������ش��������⣺
ijͬѧӦ����ͼ��ʾװ���о����ʵ����ʣ���������X����Ҫ�ɷ�������������������������ˮ��������ش��������⣺