��Ŀ����
7������β����NOx�����������������������㷺��ע����1��ij��ȤС����������������Ϣ��
N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol
2H2��g��+O2��g���T2H2O��g������H=-483.6kJ/mol
��Ӧ2H2��g��+2NO��g���T2H2O��g��+N2��g����H=-664.1kJ/mol��
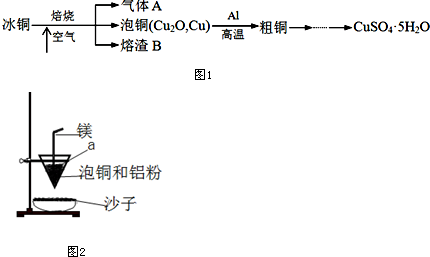
��2����С�����õ绯ѧԭ���������ͼ1װ�ý���H2��ԭNO��ʵ��[�����ӵ����Ե�SCY�մɣ��ܴ���H+��Ϊ���ʣ������ٱ�Ĥ���缫]���ٵ缫AΪ�������缫��ӦʽΪ2NO+4H++4e-=N2 +2H2O��
��3����������ԭNOԭ�����£�
����Ӧ��4NO��g��+4NH3��g��+O2��g��?4N2��g��+6H2O��g�� ����H��0��
����Ӧ��4NH3��g��+3O2��g��?2N2��g��+6H2O��g��
4NH3��g��+4O2��g��?2N2O��g��+6H2O��g��
4NO��g��+4NH3��g��+3O2��g��?4N2O��g��+6H2O��g��
�й�ʵ�������ͼ2��ͼ3��ʾ���ݴ˻ش��������⣺

�ٴ���ԭNOӦ����n��NH3��/n��NO�������ֵΪ1��������n��NH3��/n��NO��С��1ʱ��NO�ѳ��ʲ��ߣ�n��NH3��/n��NO�� ����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ�
������Ӧƽ�ⳣ������ʽ��K=$\frac{{c}^{6}��{H}_{2}O��{c}^{4}��{N}_{2}��}{c��{O}_{2}��{c}^{4}��N{H}_{3}��{c}^{4}��NO��}$�������¶ȵ����ӣ�K����С��ѡ����ӡ�������С�����䡱��
��Ӱ��N2O�����ʵ��������¶ȡ�����Ũ�Ⱥ�n��NH3��/n��NO����
��4����֪25��ʱ��Ksp��BaSO4��=1��10-10��Ksp��BaCO3��=1��10-9����һ�����BaCO3��Ӧ�����ô�����0.5mol/L��Na2SO4��Һ������ϴθ���������ϴθ������Na2SO4��ҺŨ�ȵı仯��������θҺ�е�Ba2+Ũ�Ƚ�Ϊ2��10-10mol/L��
��5������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʣ�ˮ���к��е�CaSO4������Na2CO3��Һ��������Ӧ�����ӷ���ʽΪCaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
���� ��1��������֪��Ӧ��˹���ɣ��ɵ�Ŀ�귴Ӧ�ġ�H��
��2���������ٵ缫A�ķ�Ӧ����������֪������ԭ��Ӧ�������غ���ƽ���õ缫��Ӧʽ��
��3��������ͼ3��֪��n��NH3��/n��NO�� С��1ʱ��NO�ѳ��ʲ��ߣ�n��NH3��/n��NO�� ����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ��ʴ���ԭNOӦ����$\frac{n��N{H}_{3}��}{n��NO��}$�����ֵΪ1��
�ڸ�������Ӧ�ķ���ʽ��4NO��g��+4NH3��g��+O2��g��?4N2��g��+6H2O��g������ƽ�ⳣ������ʽ����Ϊ����Ӧ���ȣ����������¶�ƽ�������ƶ���K����С��
�۸���ƽ���ƶ��Ĺ��ɡ���Ӧ�ķ���ʽ��ͼ2��ͼ3��֪��Ӱ��N2O�����ʵ����أ�
��4������BaSO4���ܶȻ�Ksp=c ��Ba2+����c ��SO42-���Լ�C��SO42-������
��5��CaSO4��CO32-��Ӧ����CaCO3���ӳ�����ת���Ƕȷ�����
��� �⣺��1��������֪��Ӧ��N2��g��+O2��g��=2NO��g����H1=+180.5kJ/mol-----��
2H2��g��+O2��g��=2H2O��g����H2=-483.6kJ/mol-----��
���ݸ�˹���ɣ���Ӧ2H2��g��+2NO��g��=2H2O��g��+N2��g�����ɢ�-�ٵõ������H=��H2-��H1=-664.1kJ/mol��
�ʴ�Ϊ��-664.1kJ/mol��
��2�����ٵ缫A��NO ת��ΪN2 ��������ԭ��Ӧ����Ϊ������������֪��Ӧ�������������غ���ƽ���õ缫��ӦʽΪ2NO+4H++4e-=N2 +2H2O��
�ʴ�Ϊ������ 2NO+4H++4e-=N2 +2H2O
��3������ͼ3��֪��n��NH3��/n��NO�� С��1ʱ��NO�ѳ��ʲ��ߣ�n��NH3��/n��NO�� ����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ�����ԭNOӦ����$\frac{n��N{H}_{3}��}{n��NO��}$�����ֵΪ1��
�ʴ�Ϊ��1��n��NH3��/n��NO��С��1ʱ��NO�ѳ��ʲ��ߣ�n��NH3��/n��NO�� ����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ�
�ڸ�������Ӧ�ķ���ʽ��4NO��g��+4NH3��g��+O2��g��?4N2��g��+6H2O��g������ƽ�ⳣ������ʽ��K=$\frac{{c}^{6}��{H}_{2}O��{c}^{4}��{N}_{2}��}{c��{O}_{2}��{c}^{4}��N{H}_{3}��{c}^{4}��NO��}$����Ϊ����Ӧ���ȣ����������¶ȵ����ӣ�ƽ�������ƶ���K����С��
�ʴ�Ϊ��$\frac{{c}^{6}��{H}_{2}O��{c}^{4}��{N}_{2}��}{c��{O}_{2}��{c}^{4}��N{H}_{3}��{c}^{4}��NO��}$����С��
�۸��ݷ�Ӧ�ķ���ʽ��ͼ2��ͼ3��֪��Ӱ��N2O�����ʵ������� �¶ȡ�����Ũ�Ⱥ�n��NH3��/n��NO����n��NH3��/n��NO�� С��1ʱ��NO�ѳ��ʲ��ߣ�n��NH3��/n��NO�� ����1ʱ��NO�ѳ������Ӳ�������N2O�������������ӣ�
�ʴ�Ϊ���¶ȣ� n��NH3��/n��NO����
��4����C��SO42-��=0.5mol•L-1��BaSO4���ܶȻ�Ksp=c ��Ba2+����c ��SO42-��=1��10-10������C��SO42-��=$\frac{1��1{0}^{-10}}{0.5}$=2��10-10mol•L-1���ʴ�Ϊ��2��10-10��
��5��CaSO4 ת��ΪCaCO3�����ӷ���ʽΪCaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
��ƽ��CaSO4��s��?Ca2+��aq��+SO42-��aq����֪���ɼ���Na2CO3��Һ����Na2CO3��Һ��CO32-��Ca2+�������CaCO3����Ca2+Ũ�ȼ��٣�ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ���������CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
�ʴ�Ϊ��Na2CO3��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
���� ������һЩ����Ϣ�����˸�˹���ɵ�Ӧ�á��绯ѧ����ѧƽ���֪ʶ���ۺ��Խ�ǿ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1����֪��H-H����Ϊ436KJ•mol-1��N��N����Ϊ945KJ•mol-1��N-H����Ϊ391KJ•mol-1��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ��N2��g��+3H2��g��?2NH3��g����H=-93 KJ•mol-1
��2�����淴ӦN2+3H2?2NH3�ں����ܱ������н��У��ﵽƽ��״̬�ı�־�Ǣڢ�
�ٵ�λʱ��������n mo1N2��ͬʱ����3n mol H2
�ڵ�λʱ����1��N��N�����ѵ�ͬʱ����6��N-H������
��������N2��H2��NH3�����ʵ���Ϊ1��3��2
�ܳ����£����������ܶȲ��ٸı��״̬
�ݳ����£���������ƽ����Է����������ٸı��״̬
��3�������£���һ��2L���ܱ������г���2.6mol H2��1mol N2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±�����
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| C��NH3��/mol•L-1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
��4�����ǵ��ʹ�ҵ����Ҫԭ�ϣ�ij���ʳ�����Ȼʯ�����Ҫ�ɷ�CaSO4��Ϊԭ�������̬���ʣ�NH4��2SO4������֪Ksp��CaSO4��=7.10��10-5 Ksp��CaCO3��=4.96��10-9���乤���������£�

��д���Ʊ���NH4��2SO4�ķ�Ӧ����ʽ��CaSO4+��NH4��2CO3=��NH4��2SO4+CaCO3�����������й����ݼ���������Ӧ�ܷ�����ԭ����ΪKsp��CaSO4��=7.10��10-5��Ksp��CaCO3��=4.96��10-9��
| ѡ�� | �� �� | ������ | ���ӹ����жϼ����ӷ���ʽ |
| A | �����£���ˮ�������C��H+��Ϊ1��10-12 mol•L-1 | K+��Cl-��S2-SO32- | һ���ܴ������� |
| B | �μӰ�ˮ | Na+��Fe3+��Cl- | ���ܴ������� Fe3++3OH-�TFe��OH��3�� |
| C | p=1����Һ | Fe3+��I- Cl- | ���ܴ������� 2Fe3++2I-�T2Fe2++I2 |
| D | ͨ������SO2���� | K+��Na+��ClO- | ���ܴ������� 2ClO-+SO2+H2O�T2HClO+SO32- |
| A�� | A | B�� | B | C�� | C | D�� | D |
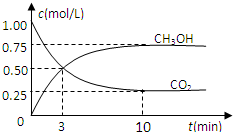 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�������䣩������1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�������䣩������1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ�� ��������ƣ�Na2S2O3�������������Լ������ﻹԭ���������ȡ������ֽ⣮��ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ����˵����a��ʢ��ϡ���b��ʢ��Na2SO3���壩�ش��������⣺
��������ƣ�Na2S2O3�������������Լ������ﻹԭ���������ȡ������ֽ⣮��ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ����˵����a��ʢ��ϡ���b��ʢ��Na2SO3���壩�ش��������⣺