��Ŀ����
17��ij�о���ѧϰС��������ϵ�֪��Ư����������Һ��Ӧ����ȡCl2����ѧ����ʽΪ��Ca��ClO��2+CaCl2+2H2SO4 $\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4+2Cl2��+2H2O���������������ʵ��������ȡCl2����֤��ijЩ���ʣ�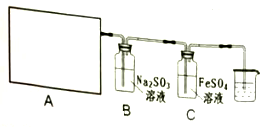
��ش�
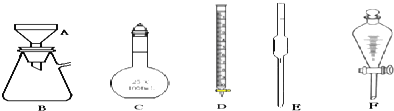
��1����ʵ����A���ֵ�װ���Ǣڣ���дװ�õ���ţ���
��2����Ҫ֤��B��Na2SO3��Һ�ѱ�����������Ҫ�Ļ�ѧ�Լ�ΪBaCl2��Һ��ϡ���
��3��ʵ�������һ��ʱ�������Ҫ̽��C��FeSO4��Һ�������ij̶ȣ���������µķ���������д���еĿհף�
| ʵ��Ŀ�� | ��������� |
| ��FeSO4��Һ��ȫδ������ | ʵ���ȡ������Һ���Թ��У���������KSCN��Һ������Һ����ɫ��ѡ�����ɫ�����ɫ������˵����Һ��ȫδ�������� |
| ��FeSO4���ֱ����� | ��ʵ��ڣ�ȡ������Һ���Թ��У�������������KMnO4��Һ������Һ��ɫ������ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ�� �죨Ѫ�죩ɫ��˵����Һ������������ |
| ��FeSO4��ȫ������ | ���ظ�ʵ����ڴ����Һ�еμ���������KMnO4��Һ����Һ����ɫ����һʵ���������ͬ�� |
1Mn${O}_{4}^{-}$+5Fe2++8H+=1Mn2++5Fe3++4H2O��
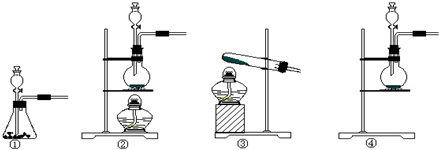
���� ��1�����ݷ�Ӧ���״̬����Ӧ����ѡ����װ�ã�
��2��������ǿ�����ԣ�������������л�ԭ�ԣ�����������������������ܷ���������ԭ��Ӧ������������ӡ������Ӻ������ӣ�����������Ʊ����������������ƣ�������������ӵļ��鷽�����鼴�ɣ��ݴ��ж�ʹ���Լ���
��3��������������ȫδ������������Һ�в����������ӣ����ݼ��������ӵķ������
���������������ֱ�����������Һ�д��������Ӻ��������ӣ������Ը��������Һ�����������ӣ������軯�ؾ��������ӣ�
��������������ȫ����������Һ�в������������ӣ��������Ը��������Һ֤���������������ӣ������軯��֤�������ӣ�
��4������KMnO4��Һ����FeSO4��Һ�У��������ӱ������������ӣ�����������ӱ���ԭ�������ӣ����ݻ��ϼ۱仯�������غ㶨����ƽ�÷�Ӧ��
��� �⣺��1���÷�Ӧ�ķ�Ӧ���ǹ����Һ�壬��Ӧ�����Ǽ��ȣ�����Ӧѡ��Һ��ϼ�����װ�ã�������ȷ��
�ʴ�Ϊ���ڣ�
��2��������ǿ�����ԣ�������������л�ԭ�ԣ�����������������������ܷ���������ԭ��Ӧ������������ӡ������Ӻ������ӣ�Cl2+SO32-+H2O=SO42-+2Cl-+2H+������������Ʊ������������������ƣ������ƺ��Ȼ����ܷ��� ��Ӧ���ɰ�ɫ�������ᱵ�������ᱵҲ�dz���������Ҫ���ų��������εĸ��ţ������Ȼ���������������ӣ�����ʹ�õ��Լ�Ϊ��BaCl2��Һ��ϡ���ᣬ
�ʴ�Ϊ��BaCl2��Һ��ϡ���
��3��������������ȫδ������������Һ�в�����Fe3+���ӣ����鷽��Ϊ��ʵ���ȡ������Һ���Թ��У���������KSCN��Һ������Һ����ɫ��˵����Һ��ȫδ��������
�ʴ�Ϊ��KSCN�����䣻
��FeSO4���ֱ�����������Һ��ͬʱ����Fe3+���Ӻ�Fe2+�������Ը��������Һ�����������ӣ������軯�ؾ��������ӣ����鷽��Ϊ��ȡ������Һ���Թ��У�������������KMnO4��Һ����Һ��ɫ����֤����Һ�к����������ӣ�����ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ��˵����Һ�к��������ӣ�֤����Һ������������
�ʴ�Ϊ���ʣ��죨Ѫ�죩��
��������������ȫ����������Һ�в������������ӣ��������Ը��������Һ֤���������������ӣ������軯��֤�������ӣ�
��4������KMnO4��Һ����FeSO4��Һ�У�MnO4-��MnԪ�صĻ��ϼ�Ϊ+7�ۣ���Ӧ��ԭΪMn2+�����ϼ۽���5�ۣ�Fe2+�Ļ��ϼ۴�+2��ΪFe3+�����ϼ�����1�ۣ������������ӵ�ϵ��Ϊ1���������ӵ�ϵ��Ϊ5����Ӧ�����������£���Ӧ����ȱ�ٵ�Ϊ�����ӣ���������ȱ�ٷֱ�Ϊ�����Ӻ�ˮ�����������غ㶨����ƽ����ƽ������ӷ�Ӧ����ʽΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��1��5��8H+��1��5Fe3+��4H2O��
���� ���⿼��������ʵ�鷽������������۷�������Ŀ�Ѷ��еȣ��漰װ�õ�ѡ�á�������ԭ��Ӧ����ƽ������ʵ�鷽������Ƶ�֪ʶ��ע����������ʵ�鷽�����������ԭ������������ѧ���ķ�����������ѧʵ��������

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�| A�� | ���ɫ��Һ������Fe��OH��3���壬���ݵijɷ���H2 | |
| B�� | ���ɫ������Fe��OH��3��������ɫ��������Fe | |
| C�� | ��ȥ���ɫ��Һ������������ʵ�鷽���Dz��� | |
| D�� | ����Fe3+����Һ�б�ˮ���Ӱ�Χ�γ�[Fe��H2O��4]3+��������������Fe3+�ĽӴ���Ӧ������ֻ���������ĵ����� |
| A�� | ʯ̼��Ľṹ��ʽ��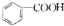 | B�� | 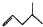 �����ƣ�2-����ϩ �����ƣ�2-����ϩ | ||
| C�� |  �ĵ��壺 �ĵ��壺 ��HCHO ��HCHO | D�� | �ǻ��ĵ���ʽ��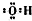 |
| A�� | ��Ԫ�صĵ����ڳ����¸�ˮ��Ӧ�����ƾ��� | |
| B�� | ��Ԫ�ص�ԭ�Ӱ뾶�ȼص�ԭ�Ӱ뾶С | |
| C�� | ��Ԫ�ص�̼����������ˮ | |
| D�� | ��Ԫ������������ˮ������ʹAl��OH��3�ܽ� |
| A�� | ��FeCl3������Һ��пɵõ�������ɵ�Fe��OH��3���� | |
| B�� | ���ά���к�ǿ�ĵ������������Դ�����������ͨ�Ź��� | |
| C�� | ��������Һ���������ϼ������������������ | |
| D�� | CaO��HCl��ˮ��Һ���ܵ��磬�����Ƕ����ڵ���� |
| A�� | CH3CH3�е�����̼ԭ����BF3�е���ԭ�Ӿ���ȡsp2�ӻ� | |
| B�� | ������ʯӢ�����еĹ�ԭ�Ӿ���ȡsp3�ӻ� | |
| C�� | BeCl2�е���ԭ�Ӻ�H2O�е���ԭ�Ӿ���ȡsp�ӻ� | |
| D�� | CO2�е�̼ԭ����CH2=CH2�е�����̼ԭ�Ӿ���ȡsp�ӻ� |
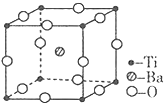 �Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺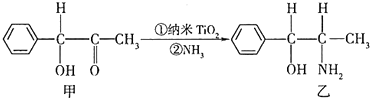
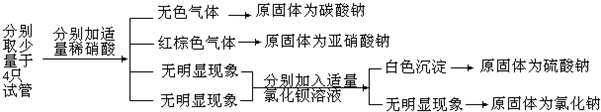 ��
��