��Ŀ����
18�����ΪԪ�����ڱ��е�һ���֣��г�11��Ԫ����Ԫ�����ڱ��е�λ�ã��û�ѧ���Żش����и����⣮| ���� ���� | �� | �� | �� | �� | �� | �� | VII | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | ��11�� |
��2���ܡ��ݡ�������Ԫ������������Ӧ��ˮ�����У������Ե���Al��OH��3�����ѧʽ��
��3��Ԫ�آڵ��⻯��ĵ���ʽΪ
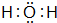 �����⻯�ﳣ���º�Ԫ�آܵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2��
�����⻯�ﳣ���º�Ԫ�آܵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2����4���ܺ͢ߵ�����������Ӧˮ����Ļ�ѧʽ�ֱ�ΪNaOH��HClO4���ܺͣ�11����Ԫ���γɻ�����Ļ�ѧʽΪNaBr�����û���������ʱ����ɫΪ��ɫ
��5����һ�����ܣ��ݣ��ޣ��������������=��������˵��ԭ��MgԪ��ԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ�
���� ��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪCl����ΪAr����ΪK����ΪCa����11��ΪBr��
��1��ͬ������ԭ�����������һ����������ͬ�������϶��µ�һ�����ܼ�С��ͬ����������ҵ縺������ͬ�������϶��µ縺�Լ�С��
��2�����������������������
��3��Ԫ�آڵ��⻯��ΪH2O��������Na��ˮ��Ӧ��������������������
��4���ܺ͢ߵ�����������Ӧˮ����ֱ�Ϊ�������ơ������ᣬ�ܺͣ�11����Ԫ���γɻ�����ΪNaBr��������Ԫ�أ���ɫ��ӦΪ��ɫ��
��5��MgԪ��ԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪCl����ΪAr����ΪK����ΪCa����11��ΪBr��
��1��ͬ������ԭ�����������һ����������ͬ�������϶��µ�һ�����ܼ�С��������Ԫ����K�ĵ�һ��������С��ͬ����������ҵ縺������ͬ�������϶��µ縺�Լ�С���ʵ縺������ΪFԪ�أ�
�ʴ�Ϊ��K��F��
��2��Al��OH��3�����ᡢ�Ӧ����������ˮ�����������������
�ʴ�Ϊ��Al��OH��3��
��3��Ԫ�آڵ��⻯��ΪH2O������ʽΪ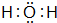 ��������Na��ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na+2H2O=2NaOH+H2����
��������Na��ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na+2H2O=2NaOH+H2����
�ʴ�Ϊ��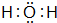 ��2Na+2H2O=2NaOH+H2����
��2Na+2H2O=2NaOH+H2����
��4���ܺ͢ߵ�����������Ӧˮ����ֱ�ΪNaOH��HClO4���ܺͣ�11����Ԫ���γɻ�����ΪNaBr��������Ԫ�أ���ɫ��ӦΪ��ɫ��
�ʴ�Ϊ��NaOH��HClO4��NaBr���ƣ�
��5��MgԪ��ԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�����һ������Mg��Al��
�ʴ�Ϊ������MgԪ��ԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ�
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ѶȲ����ضԻ���֪ʶ�Ĺ��̣�ע���Ԫ�������ɵ����⣬ע��ͬ���ڵ�һ�������쳣�����

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�| A�� | C��B��A��D | B�� | D��A��B��C | C�� | D��B��A��C | D�� | B��A��D��C |
| A�� | ���� | B�� | NaOH��Һ | C�� | Na2CO3��Һ | D�� | ���� |
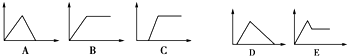 ��ͼ�У�������Ϊ��һ������ij��Һ�м���ij���ʵ�����������Ϊ���ɳ�����������ͼA��E�У�ѡ���ʺϱ��и���Ҫ������������У�
��ͼ�У�������Ϊ��һ������ij��Һ�м���ij���ʵ�����������Ϊ���ɳ�����������ͼA��E�У�ѡ���ʺϱ��и���Ҫ������������У�| ��Һ | �ӣ�ͨ�������� | ��� |
| ��1������ʯ��ˮ | ͨ�����CO2 | |
| ��2��AlCl3��Һ | ���������NH3•H2O | |
| ��3��������NaOH��Na[Al��OH��4]��Һ | ͨ�����CO2 | |
| ��4��������NaOH��Na[Al��OH��4]��Һ | ��μ���ϡ���� | |
| ��5��MgCl2��AlCl3���Һ | ��μ���NaOH��Һ������ | |
| ��6��NaOH��Һ | ��μ���AlCl3��Һ������ |

| A�� | �ͽ���������ʱ�����ڶ��� | |
| B�� | ��ŨH2SO4������170��ʱ�����ں͢ݶ��� | |
| C�� | ��Ag�����º�O2��Ӧʱ�����ٺ͢۶��� | |
| D�� | ��CH2COOH����ʱ�����ڶ��� |
| A�� | c��Cl-����c��NH4+����c��OH-����c��H+�� | B�� | c��NH4+����c��Cl-����c��OH-����c��H+�� | ||
| C�� | c��Cl-��=c��NH4+����c��H+��=c��OH-�� | D�� | c��NH4+����c��Cl-����c��H+����c��OH-�� |
| A�� | �Ѵ�ƽ��״ | B�� | δ��ƽ��״̬����Ӧ������� | ||
| C�� | δ��ƽ��״̬����Ӧ������� | D�� | ��ȷ�� |
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |