��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
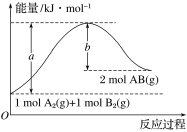
A.��ӦA(g)2B(g)��H��������Ӧ�Ļ��ΪEakJ��mol-l���淴Ӧ�Ļ��ΪEbkJ��mol-l������H=-(Ea-Eb)kJ��mol-l
B.ij�¶��£��Ȼ�����ˮ�е��ܽ����20g������¶��µı����Ȼ�����Һ���ʵ���������Ϊ20%
C.��0.2mol��L-1��CH3COOH��Һ��0.1mol��L-1��NaOH��Һ�������Ϻ���Һ���й����ӵ�Ũ���������й�ϵ��2c(H+)-c(OH-)=c(CH3COO-)-c(CH3COOH)
D.��Ũ��Ϊ0.1mol��L-1HF��Һ��ˮ����ϡ�����У�����ƽ�ⳣ��Ka(HF)���ֲ��䣬![]() ʼ�ձ�������
ʼ�ձ�������
���𰸡�C
��������
A��![]() ����Ӧ�Ļ��
����Ӧ�Ļ��![]() �淴Ӧ�Ļ��
�淴Ӧ�Ļ��![]() ����A����
����A����
B��������Һ�������������ܽ��֮��Ĺ�ϵ��![]() �����¶��µı����Ȼ�����Һ��������������С��
�����¶��µı����Ȼ�����Һ��������������С��![]() ����B����
����B����
C��![]() ��
��![]() ��Һ��
��Һ��![]() ��NaOH��Һ�������Ϻõ����ǵ�Ũ�ȵĴ���ʹ����ƵĻ������ڵ���غ㣺
��NaOH��Һ�������Ϻõ����ǵ�Ũ�ȵĴ���ʹ����ƵĻ������ڵ���غ㣺![]() �����������غ㣺
�����������غ㣺![]() ��������ʽ�õ���
��������ʽ�õ���![]() ����C��ȷ��
����C��ȷ��
D����ˮϡ�ʹٽ�HF���룬�¶Ȳ��䣬����ƽ�ⳣ�����䣬���ӽ�����ʱ��������Ũ�Ƚӽ�10-7 mol/L��������Ũ�ȼ�����С�����߱�ֵ��С����D����
��ѡ��C��

 һ����������ϵ�д�
һ����������ϵ�д�����Ŀ�������£��������ʵĵ���ƽ�ⳣ�����������˵����ȷ���ǣ� ��
HCOOH | CH3COOH | NH3��H2O | |
Ka | 1.77��10-4 | 1.75��10-5 | 1.76��10-5 |
A.Ũ����ͬ��HCOOH��Һ��NH3��H2O��Һ�������ϣ�������Һ�Լ���
B.����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ������pH��Ϊ3��HCOOH��CH3COOH��Һ��HCOOH����NaOH��Һ�������
C.0.2mol��L-1HCOOH��0.1mol��L-1NaOH�������Ϻ����Һ�У�c(HCOO-)+c(OH-)=c(HCOOH)+c(H+)
D.0.2mol��L-1CH3COONa��0.1mol��L-1����������Ϻ����Һ��(pH<7)��c(CH3COO-)>cCl-)>c(CH3COOH)>c(H+)
����Ŀ���뻯ѧƽ�����ƣ�����ƽ���ƽ�ⳣ�����������볣������k��ʾ��.�±���25���¼��ֳ�������ĵ���ƽ�ⳣ����
�� | ���뷽��ʽ | ����ƽ�ⳣ��K |
CH3COOH | CH3COOH | 1.96��10-5 |
HClO | HClO | 3.0��10-8 |
H2CO3 | H2CO3 HCO3- | K1=4.4��10-7 K2=5.6��10-11 |
H2SO3 | H2SO3 HSO3- | K1=1.54��10-2 K2= 1.02��10-7 |
�ش��������⣺
��1��CH3COOH��HClO��H2CO3��HCO3-��H2SO3��HSO3-���ɿ������ᣬ����������ǿ����_________����������________________��
��2����Na2CO3��Һ��ͨ�����������������������ӷ���ʽΪ______________________����NaClO��Һ��ͨ�������Ķ��������������ӷ���ʽΪ____________________________________��
��3����25��ʱ��1.2mol/L ��NaClO��ҺpH=____________����֪��lg2=0.3����0.10mol/L��CH3COOH��Һ�е�c(H+)=______________mol/L��