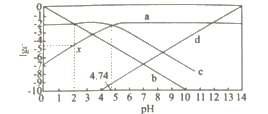
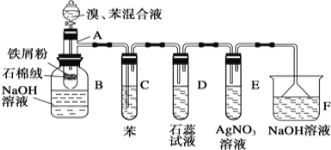
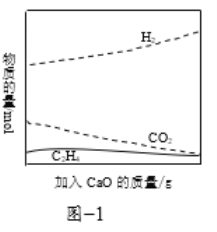
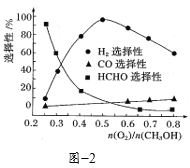
��Ŀ����
����Ŀ����ɫ��ѧ�Ի�ѧ��Ӧ�������ԭ�Ӿ�������(ԭ�ӽ�Լ)���¸��Ҫ�������ԭ�Ӿ����Է�Ӧ��ԭ�Ϸ����е�ԭ��ȫ��ת��������������������ʵ�����ŷš����м��������ұ��ķ����У�ԭ�Ӿ�������õ���(��Ӧ����һ�������½���)( )
A.![]() +C2H5Cl��
+C2H5Cl��![]() +HCl
+HCl
B.![]() +C2H5OH��
+C2H5OH��![]() +H2O
+H2O
C.![]() +CH2=CH2��
+CH2=CH2��![]()
D.![]() ��
��![]() +HBr��
+HBr��![]() +H2��
+H2��![]()
���𰸡�C
��������
������ɫ��ѧ�Ķ����֪��ԭ�Ϸ����е�ԭ��ȫ��ת��������������������ʵ�����ŷţ��Դ˽����⡣
A���÷�Ӧû��ȫ��ת��Ϊ�ұ�������HCl���ɣ���A����
B����Ӧ��û��ȫ��ת��Ϊ�ұ�������ˮ���ɣ���B����
C����Ӧ��ȫ��ת��Ϊ�ұ���û�и���Ʒ���ɣ���C��ȷ��
D����Ӧ��û��ȫ��ת��Ϊ�ұ�������HBr���ɣ��Ҳ���һ����ɣ���D����
��ѡC��

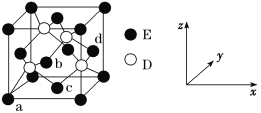
����Ŀ��̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1 MgSO4��Һ��0.5mol��L-1 NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL������ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1��5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ�����_________��
�ڲ���3����MgCO3��nH2O�����Ļ�ѧ����ʽΪ__________��
�۲���4��������Ƿ�ϴ�Ӹɾ��ķ�����__________��
��2���ⶨ���ɵ�MgCO3��nH2O�е�nֵ��
����1.000̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м�������ˮ��������ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4��5h����Ӧ���ڽ��¶�����30�棬����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
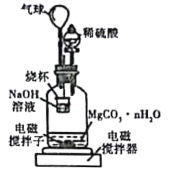
��ͼ�������������_________��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ����______��
�۲��ÿ7.8000g̼��þ���������״����CO2Ϊ1.12L����nֵΪ_______��
��3��̼��þ���������þ���ã�Ϊ�ⶨij��þ����Ҫ�ɷ���̼��þ��������̼���������������裩�����ĺ�������ʵ���ҷֱ��ȡ12.5g��þ����Ʒ���ڹ�����ϡ���Ტ��ȫת�Ƶ���ƿ�У�����ָʾ������0.010mol/L H2O2��Һ���еζ���ƽ�вⶨ���顣����H2O2��Һ��������������ʾ��
ʵ���� | 1 | 2 | 3 | 4 |
����H2O2��Һ���/mL | 15.00 | 15.02 | 15.62 | 14.98 |
��H2O2��ҺӦװ��_________��������ʽ��������ʽ�����ζ����С�
�ڸ��ݱ������ݣ��ɼ������þ������Ԫ�ص���������Ϊ_________ %������С�������λ����