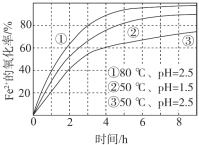
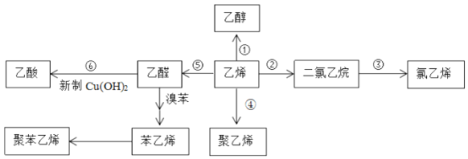
��Ŀ����
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ���������(��������δ���)��
(1)�Ҵ��������ᷴӦ�����й���ζ�����ʣ�������Ϊ____________��
(2)д����Ӧ���ͣ���_____________����______________��
(3)��Ӧ�Ļ�ѧ����ʽ��____________________________________��ʵ��������______________________________��
(4)��Ӧ����KOH���Ҵ���Һ������������������ϩ�ķ���ʽΪ��____________________________________________________________________��
(5)������ϩ�����������ŵ�����_____________________________________��
(6)����ϩ�ϳɾ۱���ϩ�Ļ�ѧ����ʽ��__________________________________________��
(7)���Ҵ��ͱ���ϩΪԭ�Ϻϳ��л���![]() ��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A
��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B
B![]()
![]() Ŀ�����)
Ŀ�����)
���𰸡��������� ��ȥ��Ӧ ������Ӧ CH3CHO+2Cu(OH)2![]() Cu2O��+CH3COOH+2H2O ��ש��ɫ�������� ClCH2CH2Cl+KOH
Cu2O��+CH3COOH+2H2O ��ש��ɫ�������� ClCH2CH2Cl+KOH![]() CH2=CHCl+H2O+KCl ��ԭ�ӡ�̼̼˫��
CH2=CHCl+H2O+KCl ��ԭ�ӡ�̼̼˫��  CH3CH2OH
CH3CH2OH ![]() CH3CHO
CH3CHO![]() CH3COOH��
CH3COOH��
��������
�ɺϳ�·��ͼ��֪����ϩ��һ�������º�ˮ�ӳ������Ҵ�����ϩ�������ӳɵõ�1��2-�������飬1��2-�������鷢����ȥ��Ӧ�õ�����ϩ����ϩ��һ�������·����Ӿ۷�Ӧ���ɾ���ϩ����ϩһ����������������ȩ����ȩ�����Ƶ�Cu(OH)2��Ӧ���������ȩ���屽���ಽ��Ӧ��ȡ����ϩ������ϩ�ۺ���ȡ�۱���ϩ���ݴ˽��
(1)�Ҵ��������ᷴӦ�����й���ζ�����ʣ�������Ϊ����������
�ʴ�Ϊ������������
(2)��Ӧ��Ϊ1��2-�������鷢����ȥ��Ӧ�õ�����ϩ����Ӧ��Ϊ��ϩһ����������������ȩ��
�ʴ�Ϊ����ȥ��Ӧ��������Ӧ��
(3)��Ӧ��Ϊ��ȩ�����Ƶ�Cu(OH)2��Ӧ�������ᣬ��ѧ����ʽ��CH3CHO+2Cu(OH)2![]() Cu2O��+CH3COOH+2H2O����ʵ����������ש��ɫ�������ɣ�
Cu2O��+CH3COOH+2H2O����ʵ����������ש��ɫ�������ɣ�
�ʴ�Ϊ��CH3CHO+2Cu(OH)2![]() Cu2O��+CH3COOH+2H2O����ש��ɫ�������ɣ�
Cu2O��+CH3COOH+2H2O����ש��ɫ�������ɣ�
(4)��Ӧ����KOH���Ҵ���Һ������������������ϩ��������ȥ��Ӧ����ѧ����ʽΪ��ClCH2CH2Cl+KOH![]() CH2=CHCl+H2O+KCl ��
CH2=CHCl+H2O+KCl ��
�ʴ�Ϊ��ClCH2CH2Cl+KOH![]() CH2=CHCl+H2O+KCl��
CH2=CHCl+H2O+KCl��
(5)�ɽṹ��ʽCH2=CHCl��֪�����������ŵ�������ԭ�ӡ�̼̼˫����
�ʴ�Ϊ����ԭ�ӡ�̼̼˫����
(6)����ϩ��һ�������·����Ӿ۷�Ӧ���ɾ۱���ϩ����ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
(7)���Ҵ��ͱ���ϩΪԭ�Ϻϳ��л���![]() ���ȱ����������
���ȱ���������� �������ϳ�·�߿������Ϊ��CH3CH2OH
�������ϳ�·�߿������Ϊ��CH3CH2OH ![]() CH3CHO
CH3CHO![]() CH3COOH��
CH3COOH��
�ʴ�Ϊ��CH3CH2OH ![]() CH3CHO
CH3CHO![]() CH3COOH��
CH3COOH�� ��
��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�