��Ŀ����
����Ŀ��25��C���ı�0.01 mol��Lһ1 CH3COONa��Һ��pH.��Һ��c��CH3COOH����c��CH3COO-����c��H +����c��OH- ���Ķ���ֵlgc����ҺpH�ı仯��ϵ��ͼ��ʾ������������ȷ����
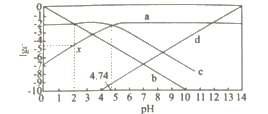
A.ͼ������������c��CH3COOH��+c��CH3COO-��=c��Na+��
B.0.01 mol��L-1CH3COOH��pHԼ������a����c���㴦�ĺ�����ֵ
C.��ͼ����Ϣ�ɵõ�x��������ֵΪ4.74
D.25��Cʱ��![]() ��ֵ�� pH�����������
��ֵ�� pH�����������
���𰸡�C
��������
������ǿ����c(CH3COO��)��c(OH��)����c(CH3COOH)��c(H +)��С����pH =7ʱc(OH��) = c(H +)�����a��b��c��d�ֱ�Ϊc(CH3COO��)��c(H +)��c(CH3COOH)��c(OH��)��
A. ���ͨ������NaOHʵ�֣���c(Na+)���ӣ���A����
B. ��a����c���㴦��c(CH3COO��)= c(CH3COOH)�����![]() ������0.01 molL1 CH3COOH��
������0.01 molL1 CH3COOH��![]() ��
��![]() ��pH = 3.37����B����
��pH = 3.37����B����
C. ��ͼ����Ϣ�ɵõ�x��lg(CH3COOH) = 2��pH = 2��![]() ��
��![]() ��lg(CH3COO��) =4.74�����x��������ֵΪ4.74����C��ȷ��
��lg(CH3COO��) =4.74�����x��������ֵΪ4.74����C��ȷ��
D. 25��Cʱ��![]() ��Ka��Kw��ֵ��pH����������䣬��
��Ka��Kw��ֵ��pH����������䣬��![]() ���䣬��D����
���䣬��D����
������������ΪC��

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ�~����Ԫ�أ��ش��������⣺
�������� | I A | II A | III A | IV A | V A | VI A | VII A | 0�� |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� |
(1)Ԫ�آٵ�������______��Ԫ�آ������ڱ�������λ��________��
(2)Ԫ�آڵļ��⻯��ĽṹʽΪ________��
(3)�õ���ʽ��ʾԪ�آ���ߵĻ�������γɹ��̣�__________________��
(4)��ʾ����ᰴԭ�Ӹ�����1:1�γɵĻ�����ĵ���ʽ��______���û������к��еĻ�ѧ��������______��
(6)�ܡ��ޡ��ߡ�������Ԫ���γɵļ����ӣ����Ӱ뾶�ɴ�С��˳����_____(�������ӷ��ű�ʾ)��