��Ŀ����
������أ�KMnO4����һ�ֳ��õ���������
I����1�������б仯�� ���ҳ�����һ���仯�롰
���ҳ�����һ���仯�롰 �����һ����Ӧ��д���÷�Ӧ�����ӷ���ʽ____ ��
�����һ����Ӧ��д���÷�Ӧ�����ӷ���ʽ____ ��
��2����ͬ�����¸�����ؿɷ������·�Ӧ��
 �ɴ˿�֪������������ӣ�MnO��4����Ӧ��IJ����� �йء�
�ɴ˿�֪������������ӣ�MnO��4����Ӧ��IJ����� �йء�
��3�����������Һ�������������·�Ӧ��
��������Ӧǰ����������������2.8g������Ԫ����KMnO4֮�䷢������ת�Ƶ���ĿΪ ����
�ƺ�ݳ��ѳ�Ϊһ��������⡣����ʻ��Ա�����оƾ�Ũ�ȣ�BrAC���ķ����ж��֡�
��4�����������ü���Լ���ɫ�仯�����ж�BrAC���������·�Ӧ���BrAC:
��ɲ���ƽ������Ӧ��
��5����������������������������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ���÷�Ӧ�Ļ�ѧ����ʽΪ____ ��
14��
��2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O ��3�֣�
����Һ����� ��2�֣�
��0.1NA ��6.02��1022�� ��3�֣�
��3CH3CH2OH+2KMnO4=3CH3CHO+2MnO2+2KOH+2H2O ��3�֣�
��I2O5+5 CH3CH2OH��I2+5CH3CHO+5H2O ��3�֣�
��������������š�MnO4-��Mn2+���ǻ�ԭ����ô��һ���̱ض�����������ֻ�С�C2O42-��CO2�������⣬����ʽΪ2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O��
���������е������뷴Ӧ��֪����Һ����ԡ�
���ɻ�ѧ����ʽ�ɵù�ϵʽ��10FeS ~ 10S ~ ��m���̣�~ ת��e-
880g 320g 560g 20mol
2.8g ��0.1mol
ת�Ƶ�����ĿΪ0.1NA ����6.02��1022����
�����ڷ�Ӧ�����˼���ݵڢ����֪KMnO4����ԭΪMnO2���ʷ���ʽΪ��
3CH3CH2OH+2KMnO4=3CH3CHO+2MnO2+2KOH+2H2O
��������������ĵ�����Һ���BrAC���Ҵ�������Ϊ��ȩ����ôI2O5�ͱ���ԭΪI2��
I2O5+5 CH3CH2OH��I2+5CH3CHO+5H2O
���㣺������ԭ��Ӧ�Ļ���֪ʶ��������ԭ��Ӧ����ʽ����д����ƽ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�Ϊ�˷�ֹǹ֧���⣬����ǹ֧�ĸ����������NaNO2��NaOH�Ļ��Һ�н��л�ѧ����ʹ���������������Fe3O4�����ܵı����㡪����������������̿������л�ѧ����ʽ��ʾ��
��3Fe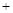 NaNO2
NaNO2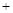 5NaOH �� 3Na2FeO2
5NaOH �� 3Na2FeO2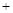 H2O
H2O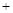 NH3��
NH3��
�� Na2FeO2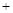 NaNO2
NaNO2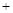 H2O �� Na2Fe2O4
H2O �� Na2Fe2O4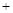 NH3��
NH3��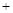 NaOH
NaOH
��Na2FeO2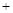 Na2Fe2O4
Na2Fe2O4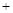 2H2O
2H2O Fe3O4
Fe3O4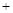 4NaOH
4NaOH
��1����ƽ��ѧ����ʽ�ڡ�
��2��������Ӧ���л�ԭ��Ϊ ������ԭ���� ������1mol Na2FeO2���ɣ���Ӧ���� mol����ת�ơ�
��3�������γɡ��������Ĺ��̣�����˵����ȷ���ǡ��������������������������������� ��
| A�����������̲��������Ⱦ | B����Ӧ�����ɵ��������������п���ʴ���� |
| C����Ӧ�٢ڢ۾���������ԭ��Ӧ | D����Ӧ�٢��е���������ΪNaNO2 |
Na2SO3�ڿ������ױ����������ʡ�ijͬѧΪ֤��Na2SO3�л�ԭ�ԣ���һƿʵ���ҳ��ڴ�ŵ�Na2SO3������ȡ����������ˮ������һ�������ռ���Һ��������ˮ������Һ��Ϊ��ɫ��
��1���ڼ�����Һ��Br2��Na2SO3��Ӧ�����ӷ���ʽ ��
��2����Ӧ�����Һ����SO32-��SO42-��Br-��OH-�������ӣ��±���ijͬѧ��������SO32-��SO42-��Br-��ʵ�鱨�棬�����δ����IJ��֡�
��ѡ�Լ���2 mol��L-1HCl��1 mol��L-1 H2SO4��l mol��L-1BaCl2��l mol��L-1Ba(NO3)2��1 mol��L-1 KMnO4��CCl4�����Ʊ�����ˮ��Ʒ����Һ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ�����Թ��У��������2mol��L-1HCl���ٵμ�����1 mol��L-1BaCl2 ��Һ�� | �а�ɫ�������ɣ�֤������Һ�к��С�SO42- �� |
| ����� | | |
| ����� | | |
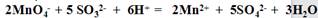
�ظ��������Σ�ÿ������0.10mol/LKMnO4��Һ����ֱ�Ϊ20.02 ml�� 20.00 ml��19.98 ml�������ԭ������Na 23 S 32 O 16)
�ټ�����Ʒ��Na2SO3����������Ϊ �����������3λ��Ч���֣�
�ڲ���ʱ����δ��0.10mol/L������KMnO4��Һ��ϴ�ζ��ܣ��ᵼ�²ⶨ��� �����ƫ�ߡ�����ƫ�͡���û��Ӱ�족��
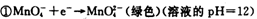


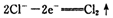

 2Fe(s)��3CO(g) ��H����492.7kJ/mol
2Fe(s)��3CO(g) ��H����492.7kJ/mol