��Ŀ����
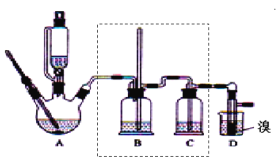
����Ŀ��ʵ�����Ʊ�1��2���������飬�����������Ҵ����Ʊ���ϩ��������ϩ�����������Ʊ�1��2���������飬װ����ͼ��ʾ���й������б������ʾ���ش��������⣺
�Ҵ� | 1��2���������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ�/gcm-3 | 0.79 | 2.2 | 0.71 |
�е�/�� | 78.5 | 132 | 34.6 |
�۵�/�� | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����__��
a.������Ӧ b.�ӿ췴Ӧ�ٶ�
c.��ֹ�Ҵ��ӷ� d.���ٸ�������������
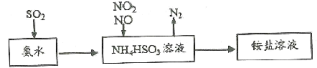
��2����װ��A�г���Ũ������Ҵ��⣬��Ӧ����__����Ŀ����__��װ��A�����ɸ��������ѵĻ�ѧ��Ӧ����ʽΪ__��
��3��ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2��Ϊ����֤SO2�Ĵ��ڲ���ȥSO2�Ժ�����Ӧ�ĸ��ţ�ijͬѧ��A��D֮�������B��C����װ�ã�����B��C�пɷֱ�ʢ��___��
a.����KMnO4��ˮ b.Ʒ���NaOH��Һ
c.����KMnO4��NaOH��Һ d.Ʒ�������KMnO4
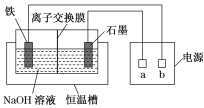
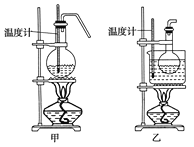
��4���ס�����װ�þ�������ʵ��������ˮ�Ҵ���ȡ��ϩ����ͼ���ø���ԡ����(���ͷе�290�棬�۵�18.17��)���������¶ȴﵽ��Ӧ�¶�ʱ����ʢ����ˮ�Ҵ���Ũ������Һ����ƿ��������У��ܿ�ﵽ��Ӧ�¶ȡ��ס�����װ����Ƚϣ���װ������Щ�ŵ�__��д����ʵ��������ˮ�Ҵ���ȡ��ϩ�Ļ�ѧ����ʽ___��
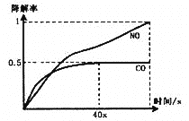
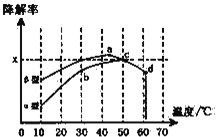
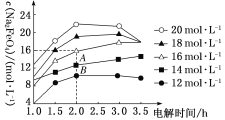
��5����1��2����������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ú���Ӧ��__�㣻�����������������������ѡ�����__�ķ�����ȥ��
���𰸡�d ��ʯ�����Ƭ��ֹ���� ��ֹ���� 2CH3CH2OH![]() C2H5��O��C2H5+H2O b �����ڿ����¶ȣ����Ⱦ��ȣ����ٸ���Ӧ�ķ��� CH3CH2OH
C2H5��O��C2H5+H2O b �����ڿ����¶ȣ����Ⱦ��ȣ����ٸ���Ӧ�ķ��� CH3CH2OH![]() CH2=CH2+H2O �� ����
CH2=CH2+H2O �� ����
��������
��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�